Novartis Pharmaceuticals Corporation (“Novartis”), in cooperation with the U.S. Consumer Product Safety Commission (“CPSC”) is implementing a CPSC-approved corrective action plan for SANDIMMUNE® (cyclosporine capsules, USP) 100-mg soft gelatin capsules and NEORAL® (cyclosporine capsules, USP) MODIFIED 100-mg soft gelatin capsules 30-count blister packs within expiry distributed in the United States. This action is not a result of any quality or efficacy issues with the medicines for their intended use.
The blister cards in which these products are packaged do not meet child-resistant packaging requirements, posing a potential risk of harm if children open the package and swallow the medicine. SANDIMMUNE (cyclosporine capsules, USP) 25-mg soft gelatin capsules and NEORAL (cyclosporine capsules, USP) MODIFIED 25-mg soft gelatin capsules 30-count packages are not included in this corrective action.
This notification applies ONLY to the National Drug Codes (NDCs) and lot numbers below:
| Product Description | Package Description | NDC Number on Carton | NDC Number on Blister Pack | Lot Number | Expiration Date |
|---|---|---|---|---|---|
| SANDIMMUNE soft gelatin capsules 100-mg |
Blister packs of 30 capsules | 0078-0241-15 | 0078-0241-61 | APCA136 APCA339 APCA793 APCC238 |
September 2020 February 2021 January 2022 July 2022 |
| NEORAL soft gelatin capsules 100-mg MODIFIED |
Blister packs of 30 capsules | 0078-0248-15 | 0078-0248-61 | APCA437 APCA979 |
July 2020 March 2021 |
This is a CPSC-approved corrective action that is intended to reach the consumer, which means we are making consumers, pharmacists, retailers, and wholesalers aware of the packaging issue and the corrective action.
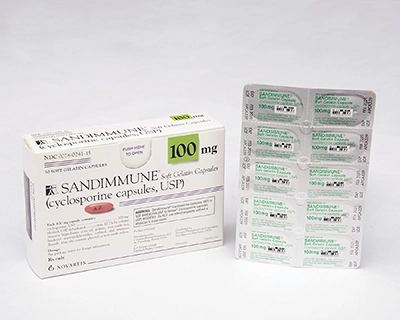
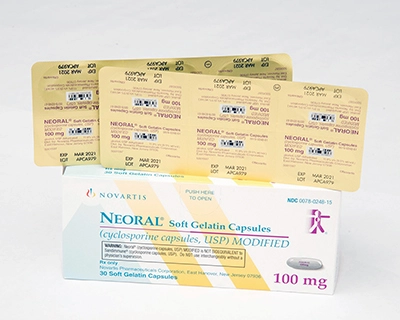
REPRESENTATIVE PHOTOS:
These images show the type of packaging involved in the corrective action for one of the affected lot numbers of each product. Please refer to the table above for a list of all affected lots.
|
|
| SANDIMMUNE 100-mg soft gelatin capsules | NEORAL 100-mg soft gelatin capsules MODIFIED |
PATIENT ACTION:
Anyone having the packages listed above should call 1-866-629-6182 from 8:00 AM to 8:00 PM ET Monday through Friday and 8:00 AM to 8:00 PM ET Saturday and Sunday or order via email at [email protected] to receive a resealable child-resistant pouch free-of-charge in which to store the blister packs within your possession.
The quality and efficacy of these products have not been compromised, so patients should continue taking their medicine as directed by their physician. However, due to the risk of harm if children open the package and swallow the medicine, immediately secure this medicine so that it is out of the sight and reach of children.
Instructions for using the child-resistant pouch are on the pouches themselves, and the following video demonstrates their use.
Further information about this corrective action is available by calling Novartis at 1-866-629-6182.
Any medical-related inquiries should be directed to Novartis Pharmaceuticals Corporation at 1-888-NOW-NOVA (1-888-669-6682). Please report any adverse events by calling Novartis at the same phone number, or by e-mailing [email protected]. Adverse events can also be reported to the FDA online at www.fda.gov/medwatch/report.htm.