Key Releases are ad hoc announcements pursuant to SIX Swiss Exchange Article 53 Listing Rules.
Header
News Archive
News Archive Navigation
icon
News Archive Navigation Language
Language Preferences
Showing 1840 results
October 2015
-
Media Release
FDA accepts Sandoz regulatory submission for a proposed biosimilar etanercept
Etanercept is an anti-TNF medicine used to treat a range of immunological diseases including rheumatoid arthritis and psoriasis. Sandoz is seeking approval for all indications included in the… -
Story Discovery

Novartis cancer researchers advance precision medicine
Learn how NIBR uses next generation sequencing to transform clinical trials and diagnostics in oncology.
September 2015
-
Media Release
Novartis announces NEJM publication of secukinumab Phase III data confirming significant efficacy in patients with psoriatic arthritis
In the FUTURE 1 study, secukinumab showed rapid and significant efficacy in active psoriatic arthritis (PsA) patients, including improvement of skin and joint disease and reduction in progression of… -
Story Patient Perspectives
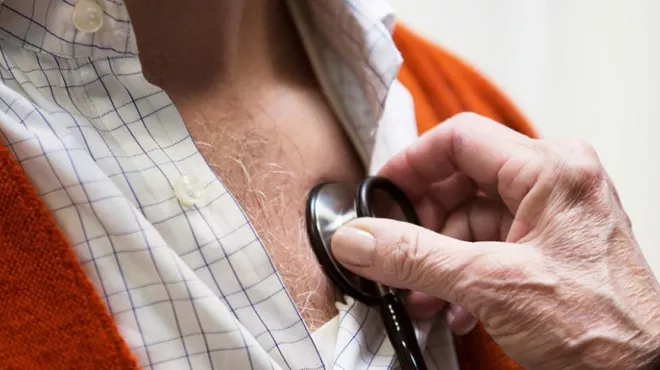
Heart failure: coping with one of the world’s biggest killers
Patients are learning to live with a chronic disease that will affect one in 5 people over age 40.
-
Media Release
Patients with aggressive form of melanoma lived for more than two years on average when taking Novartis therapies Tafinlar® + Mekinist®
Phase III data showed median overall survival of 25.6 months in patients with BRAF+ V600E/K metastatic melanoma who received Tafinlar + Mekinist Tafinlar + Mekinist combination also demonstrated… -
Media Release
Novartis drug Afinitor® significantly improves progression-free survival in advanced nonfunctional gastrointestinal and lung NET
In pivotal study, everolimus reduced risk of disease progression by 52%; showed 11.0-month median progression-free survival vs 3.9 months for placebo[1] Advanced, progressive, nonfunctional… -
Media Release
Novartis' new heart failure medicine Entresto(TM) recommended by CHMP for EU approval
Positive opinion from EU review body puts Entresto on track to be approved for HFrEF patients across Europe likely by year end Entresto was studied in world's largest heart failure trial which… -
Media Release
Novartis appoints James E. Bradner, MD as President of the Novartis Institutes for BioMedical Research as Mark Fishman retires
New leader to continue to drive long-term innovation in Novartis research in wide range of therapeutic areas Basel, September 24, 2015 - Novartis announced today that Dr. James (Jay) E.… -
Media Release
Novartis nomme le Dr James E. Bradner Président des Instituts Novartis pour la Recherche Biomédicale suite au prochain départ à la retraite de Mark Fishman
Un nouveau responsable pour continuer de stimuler l'innovation à long terme au sein de la recherche chez Novartis dans des domaines thérapeutiques très variés Bâle, le 24 septembre 2015 -… -
Media Release
Novartis ernennt Dr. James E. Bradner zum Präsidenten der Novartis Institutes for BioMedical Research, da Mark Fishman in den Ruhestand tritt
Neuer Leiter wird weiterhin langfristige Innovationen in zahlreichen Therapiegebieten der Novartis Forschung vorantreiben Basel, 24. September 2015 - Novartis gab heute bekannt, dass… -
Media Release
Novartis launches 'Novartis Access', a portfolio of affordable medicines to treat chronic diseases in lower-income countries
First-of-its-kind portfolio approach in healthcare industry, aiming to increase availability and affordability of 15 medicines against cardiovascular diseases, diabetes, respiratory illnesses and… -
Media Release
Novartis lance « Novartis Access », une gamme de médicaments à prix abordables pour lutter contre les maladies chroniques dans les pays à faible revenu
Une approche unique en son genre dans l'industrie de la santé, qui vise à rendre 15 médicaments plus facilement disponibles et plus accessibles pour traiter les maladies cardiovasculaires, le diabète…
Pagination
- ‹ Previous page
- 1
- …
- 137
- 138
- 139
- 140
- 141
- 142
- 143
- …
- 154
- › Next page