The CAN-COVID clinical trial has been one of the ways Novartis is drawing on its global resources and scientific expertise in response to the COVID-19 pandemic.
Click here for the CAN-COVID media release
While we are disappointed with the outcome of the CAN-COVID trial, we are proud to be part of the international effort being made to address the global pandemic. Novartis and the healthcare industry as a whole play a vital role in developing medicines and offering support to help combat COVID-19.
We are deeply grateful to the patients who participated and their caregivers, as well as the healthcare professionals and hospital staff who made the CAN-COVID trial possible while fighting the pandemic on the front line.
COVID-19 and CRS
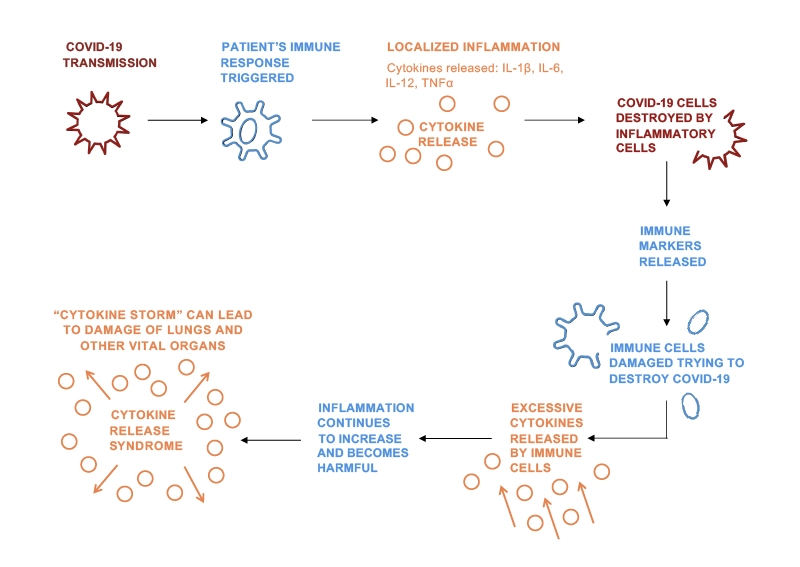
Evidence suggests that some patients who develop severe illness due to COVID-19 do so due to Cytokine Release Syndrome (CRS) – this is when the body produces too many cytokines that can cause damage rather than helping the body fight the virus. This can cause life-threatening complications in these patients.
Stages of COVID-19 and the CAN-COVID trial
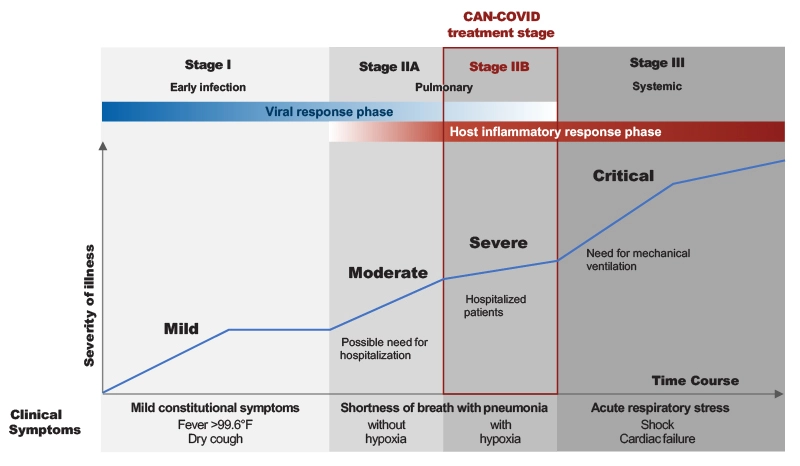
The CAN-COVID trial focused on patients during a specific stage of COVID-19 – severe COVID-19 patients with pneumonia and CRS. This includes people whose lungs are affected and have low blood oxygen levels (Stage IIB). The patients were hospitalized and hypoxic but not requiring intubation or invasive mechanical ventilation.

CAN-COVID was initiated due to evidence of elevated interleukin (IL)-1β levels in patients with COVID-19-induced pneumonia and cytokine release syndrome (CRS) However, the results are consistent with the latest evidence suggesting that targeting interleukins (such as IL-1), which form part of the body’s overactive immune response, is not likely to be beneficial in patients with severe COVID-19.