Key Releases are ad hoc announcements pursuant to SIX Swiss Exchange Article 53 Listing Rules.
Header
News Archive
News Archive Navigation
icon
News Archive Navigation Language
Language Preferences
Showing 1809 results
March 2019
-
Key Release
Novartis receives FDA approval for Mayzent® (siponimod), the first oral drug to treat secondary progressive MS with active disease
Mayzent® (siponimod) is the first and only treatment specifically approved for patients with active secondary progressive multiple sclerosis (SPMS) in over 15 years[1] Up to 80% of patients… -
Story From Our Labs
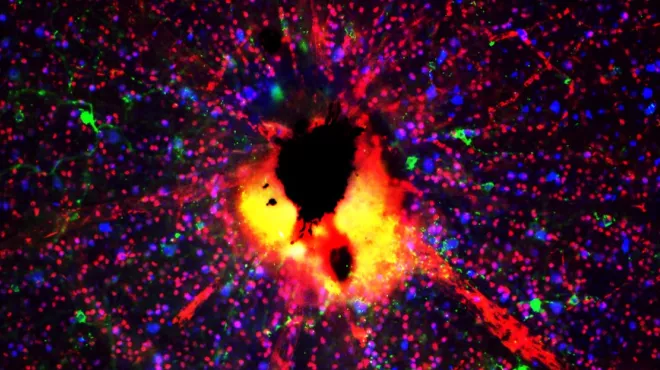
What goes wrong with the window to the soul in glaucoma?
Speaking with Ganesh Prasanna, Director of Ophthalmology at NIBR.
-
Key Release
Novartis plans for Alcon spin-off on April 9, 2019
Alcon obtained approval for listing on SIX Swiss Exchange and New York Stock Exchange Alcon will seek effectiveness of Form 20-F registration statement from the US Securities and Exchange… -
Story Discovery
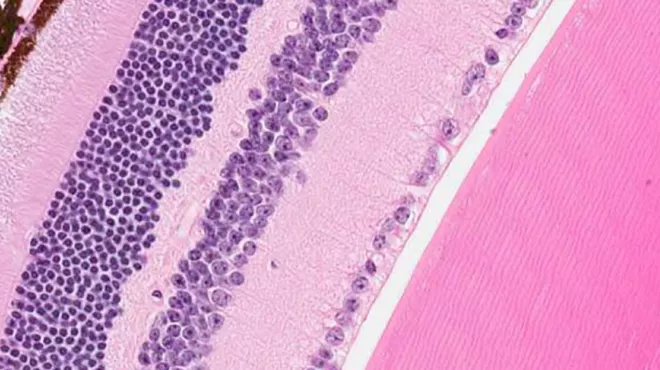
Experimental gene therapy discovered in-house enters clinical testing
Novartis is taking its investigational gene therapy for a degenerative eye disease into clinical trials.
-
In The News
It's Time to "Pivot Towards Transformational Innovation"
In this interview with Susie Gharib from Fortune, Vas Narasimhan, CEO of Novartis, shares his perspective on changing the culture within Novartis. -
Story Discovery
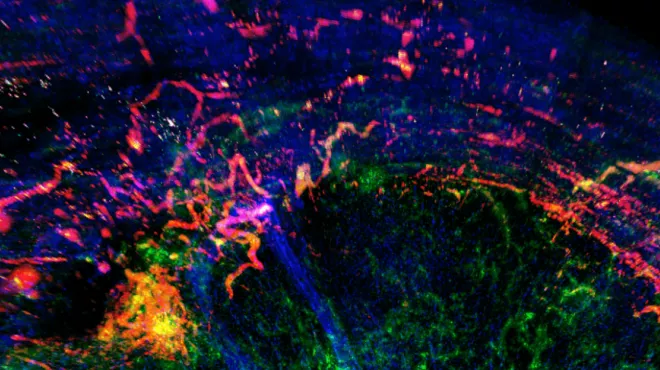
Visualizing immuno-oncology research
Learn how Novartis researchers are working to outsmart tumors.
-
Media Release
Alcon Announces Acquisition of PowerVision, Inc.
Acquisition demonstrates Alcon's commitment to driving growth and innovation in advanced technology intraocular lenses (AT-IOLS) to meet the needs of cataract surgery patients who desire… -
Media Release
Novartis joins the Global Chagas Disease Coalition and also announces first multinational, prospective, randomized study in people with chronic Chagas cardiomyopathy
Study is planned to assess efficacy and safety of Entresto® (sacubitril / valsartan) vs enalapril in 900 patients with chronic Chagas cardiomyopathy; recruitment is planned to commence within 2019… -
Featured News
Global Equal Parental Leave Policy for all Novartis parents
Fourteen weeks. That’s how many weeks all new Novartis parents will get as minimum paid parental leave, regardless of gender, rolling out from July 1, 2019.
-
Key Release
Novartis announces change in Sandoz leadership
Richard Francis, CEO Sandoz, to step down on March 31, 2019 Francesco Balestrieri, Head Region Europe, Sandoz, appointed ad-interim CEO Sandoz Basel, March 14, 2019 - Novartis announced… -
Media Release
Three winners of 2019 Sandoz Healthcare Access Challenge (HACk) are announced at SXSW
Sandoz commits to increasing support from one winner to three, following impressive pitches from the finalists Winning ideas proposed innovative uses of artificial intelligence, mobile apps and…
Pagination
- ‹ Previous page
- 1
- …
- 73
- 74
- 75
- 76
- 77
- 78
- 79
- …
- 151
- › Next page