In response to dry soil, a sensor in a garden triggers a sprinkler to deliver a shower of rain. Disaster strikes, though, if the sensor gets stuck in the “on” position. The shower turns into a stalled storm that floods the garden.
A similar problem can occur in the body. Immune cells contain danger sensors. They respond to danger with a sprinkling of signals that trigger healing inflammation. But if the danger sensor gets stuck on, the result is chronic inflammation that can cause more damage than good.
Novartis researchers aim to break this pattern by silencing part of the body’s danger-sensing system, particularly in the case of chronic disease. This system, driven by a molecular machine called the inflammasome, is gaining attention from drug hunters because blocking it could potentially help stop damaging inflammation where it starts. This precise approach to lowering levels of inflammation in the body is part of a wider effort to find new ways to treat chronic diseases from Alzheimer’s disease and cancer to osteoarthritis and gout.
The idea to target the inflammasome seems sensible, but it would have been impossible just two decades ago. Back then, no one knew this danger sensor existed.
 VIDEO
VIDEO
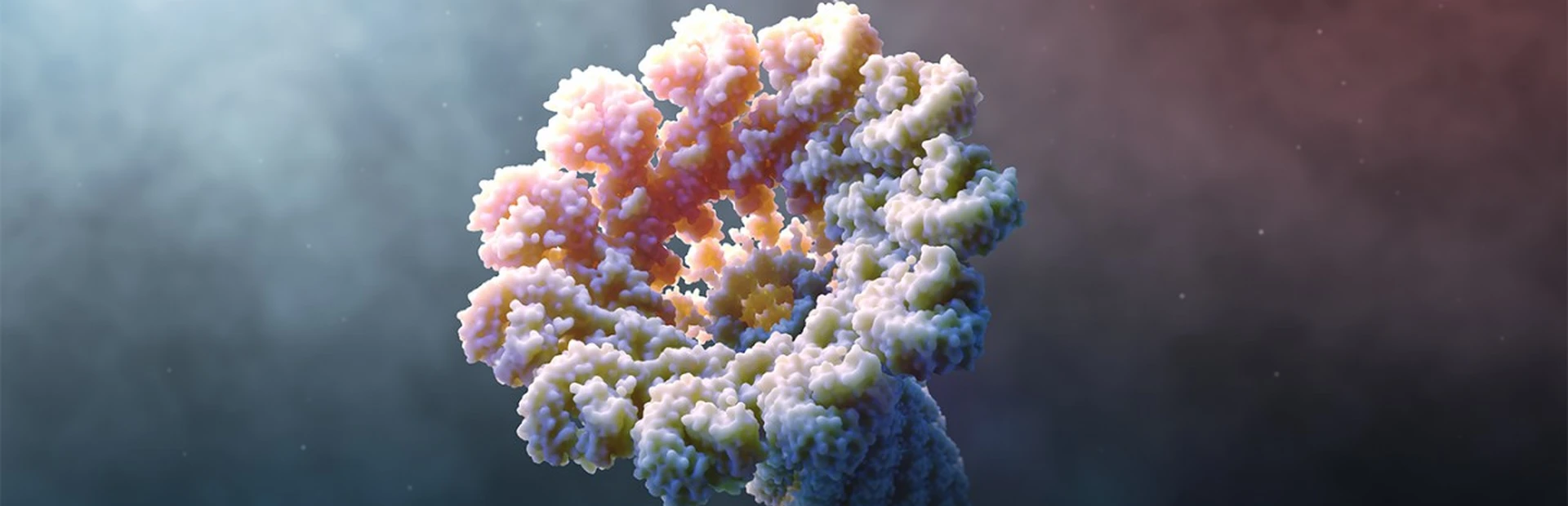
What is the inflammasome? Animation by Mark Mazaitis.
“This is one of the oldest machines in biology from an evolutionary perspective, and we’ve only recently just learned of it,” says Guido Junge, a clinical expert in inflammatory diseases at the Novartis Institutes for BioMedical Research (NIBR). “We suspect it’s a powerful player in many diseases.”
A powerful system
In 2001, scientists in the US learned about a mutation in the gene that codes for a protein called NLRP3. At the time, they knew only one thing about NLRP3: Mutations that alter it are devastating for the people who have them.
“It was a dream come true to find this gene,” says Dan Kastner, an inflammatory disease expert and scientific director at the US National Human Genome Research Institute, who in the late 1990s uncovered the genetic roots of related illnesses. “Although there was speculation that it might somehow regulate inflammation, no one knew what it did. So the idea of a treatment was still just a dream.”
If untreated, children with NLRP3 mutations have near-constant fevers, chills and joint pain. Chronic inflammation takes a toll and their lives are often cut short.
Not long after the discovery of NLRP3, researchers linked it to the inflammasome, and more research linked the inflammasome to an inflammatory signal called IL-1 beta. That signal became a guiding star: Elevated levels of IL-1 beta are a sign of chronic inflammation.
“You’re looking at tons of data and suddenly you see a pattern,” says Junge. “A signal that could help guide therapies.”

Today, medicines that block IL-1 beta enable patients with NLRP3 mutations to live longer and livelier.
“The inflammasome is a very powerful system that can be modulated to great benefit,” says Kastner. “Those successes give us hope for benefit in more subtle situations, such as chronic illness.”
Chronic disease and a runaway danger sensor
Researchers eventually figured out that NLRP3 is a danger sensor. The human body has 24 of these danger sensors, most of which notice bacteria and viruses.
The NLRP3 sensor is different. It recognizes crystals, protein aggregates and unhealthy cellular stress.
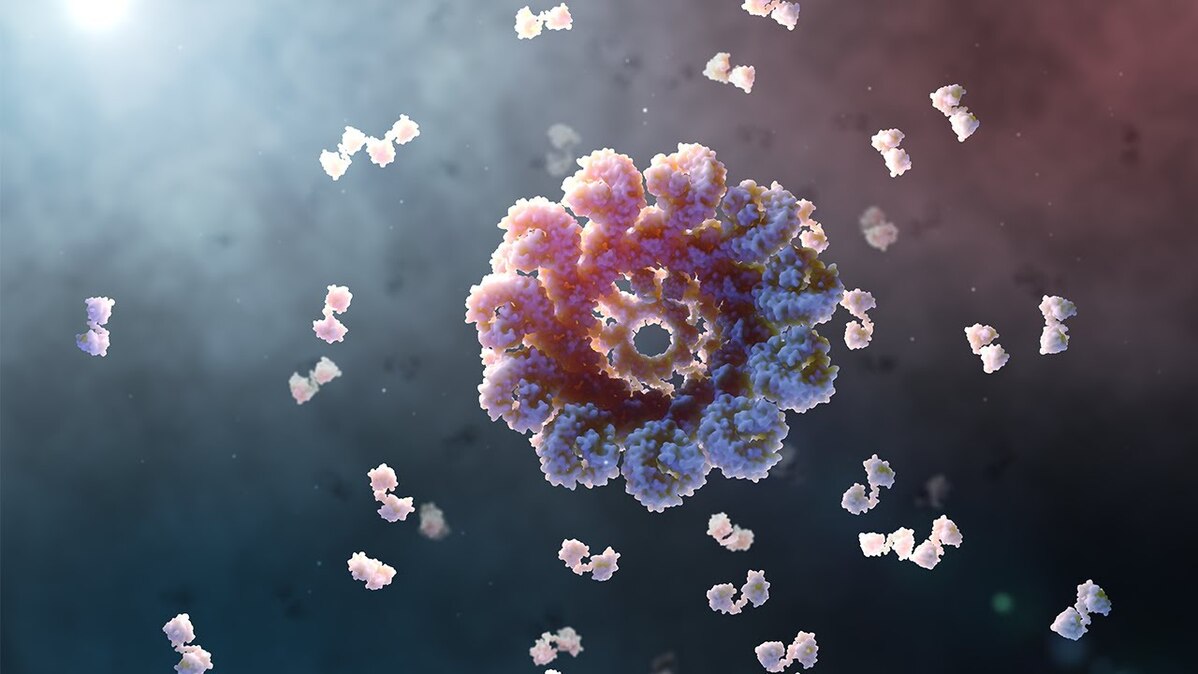
When it senses these dangers, it self-assembles into an inflammasome, which resembles a microscopic ray gun. Its job is to send out flares that alert the rest of the immune system to launch an inflammatory response in an attempt to resolve the problem.
Trouble occurs when the NLRP3 sensor gets stuck in the “on” position. This can be caused by mutations, but researchers are learning that chronic diseases can also cause the NLRP3 sensor to constantly sense danger.
In Alzheimer’s disease, for example, plaques in the brain can trip the sensor. So can hardened arteries in patients with cardiovascular disease and crystals that occur in patients with gout. A tripped sensor can create a constant state of inflammation.
“If we have a chronically alerted immune system, that’s not good,” says Joerg Eder, an immunology expert at Novartis. “People think the problem is the disease, but the damage to the tissue may also come from the immune system.”
In cancer, for instance, tumor cells themselves appear as danger signals and trigger the inflammasome. The resulting inflammation ends up promoting tumor growth rather than inducing an assault on the tumor. As a result, the cancer cells persist and continue to appear as danger signals.
“Chronic disease can cause a vicious cycle of inflammation and damage and more inflammation,” says Junge.
The NLRP3 inflammasome may also be involved in some cases of COVID-19. Some people with COVID-19 experience a runaway immune reaction to the coronavirus.
Doctors call the effect a “cytokine storm” because the immune system floods the lungs with inflammatory signals such as IL-1 beta. It is an example of a shower of immune signals in response to viral damage turning into a stalled and raging storm. Scientists around the world are investigating existing medicines that quiet the immune system to see if they might be safe and effective against COVID-19.
This is one of the oldest machines in biology... We suspect it’s a powerful player in many diseases.
Guido Junge
Damping the danger sensor
Several companies, including Novartis, have identified a range of ways to interrupt the NLRP3 inflammasome. There are many viable approaches, from blocking the signals the inflammasome produces to preventing the inflammasome from assembling in the first place.
Novartis researchers discovered a way to block NLRP3 activation. Simultaneously, researchers working at a company called IFM Tre made a similar discovery. The approaches turned out to be complementary, achieving the same anti-inflammatory goal in different ways.
Novartis acquired IFM Tre in 2019 and is the first company to have an anti-NLRP3 therapy in early-stage human clinical trials, though it certainly won’t be the last. There is much to be learned about how patients with chronic diseases respond to this approach.
“This is our great task ahead of us,” says Eder. “To find those chronic inflammatory settings where the NLRP3 is triggered and begin testing our hypothesis in patients.”
Hero image by Mark Mazaitis