Zoom in on the brain, and at every level you find an amazing degree of structure and organization. Bundles of nerves form pathways that carry signals from one part to another. Distinct layers of neurons form within the brain’s cortex (gray matter), with columns and connections spanning from one layer to the next. Such order bespeaks a tight level of control over how individual neurons divide and grow.
When that control breaks down, the resulting chaos can have dire consequences for a person’s cognitive and intellectual development.
That’s the case with tuberous sclerosis complex (TSC), a rare genetic condition. TSC patients harbor mutations in one of two genes, TSC1 and TSC2. Normally the proteins encoded by these genes work together to tamp down the mTOR complex, a central switching hub for many growth signals within neurons (and other cells as well). When TSC1 or TSC2 don’t work, however, mTOR tells neurons to continually grow and divide. The brain becomes riddled with benign growths called tubers, full of misaligned neurons and broken connections.
 VIDEO
VIDEO
Scientists in the Neuroscience group at the Novartis Institutes for BioMedical Research (NIBR) want to connect the dots from broken genes to malfunctioning cellular pathways to cell growth run amok in the brain. To do it, they’re combining induced pluripotent stem (iPS) cell technology and three-dimensional culture techniques to take donated cells from TSC patients and healthy individuals, reprogram them into neurons and generate brain organoids — miniature, organ-like structures that mimic the layered organization of cells in the brain's cortex. Think of them as “brains” in a dish.
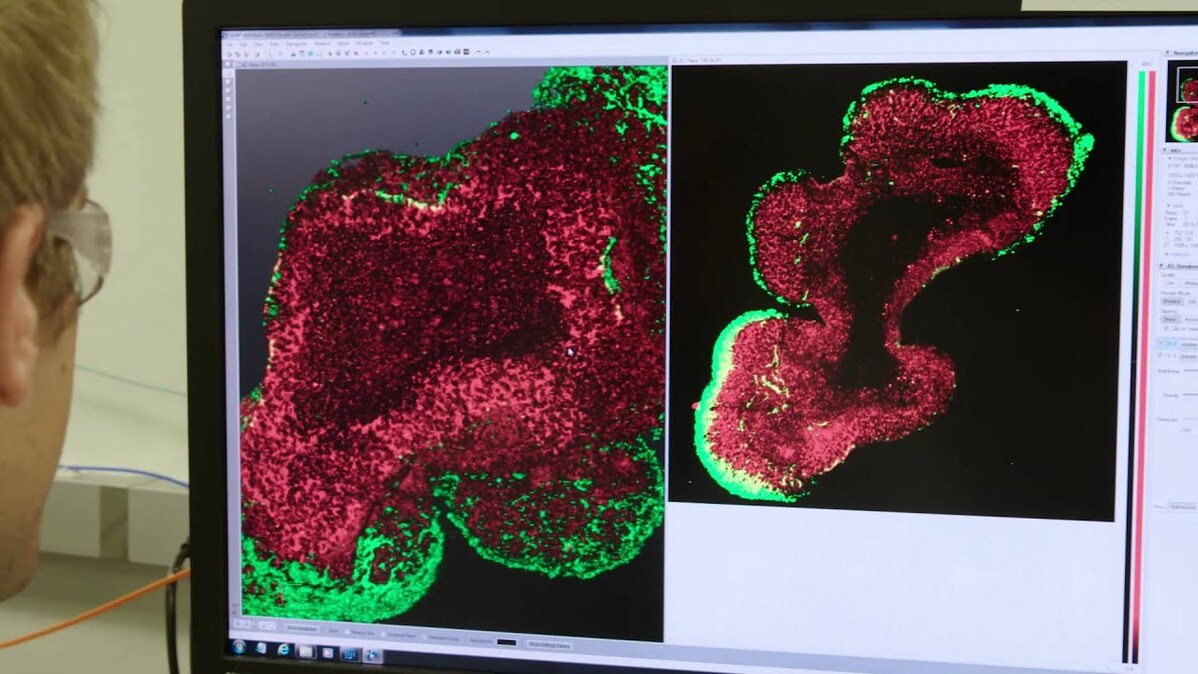
Take the organoid above, generated by NIBR neuroscientists Ajamete Kaykas and Max Salick from iPS cells that started life as cells from a healthy individual. It’s been stained to reveal the presence of a cytoskeletal protein called beta 3 tubulin, a marker unique to neurons; in this “heatmap” view, the redder an area, the more densely the neurons are packed together. At 14 days old, this organoid’s neurons are already starting to coalesce into layers similar to those seen in a developing cortex.
Organoids like this one are providing researchers like Kaykas and Salick a chance to watch TSC developmentally play out in an environment reminiscent of brain cells’ natural three-dimensional context, and potentially test prospective therapies — essentially running early stage trials in a dish. Kaykas, a senior investigator in NIBR’s Neuroscience group, is particularly excited about the opportunity to study the genetics of TSC and other so-called “mToropathies” (conditions arising from unchecked mTor activity) in a purely human system.
"The ability to take skin or blood cells, push those cells back an embryonic stem cell-like state and then drive them forward into nearly any cell type has been a game changer in our ability to model human disease," he says.
Learn more about the team’s work.
Image credit: Max Salick/NIBR
Three-dimensional clusters of human neurons could allow scientists to watch neurological diseases develop in the lab.



