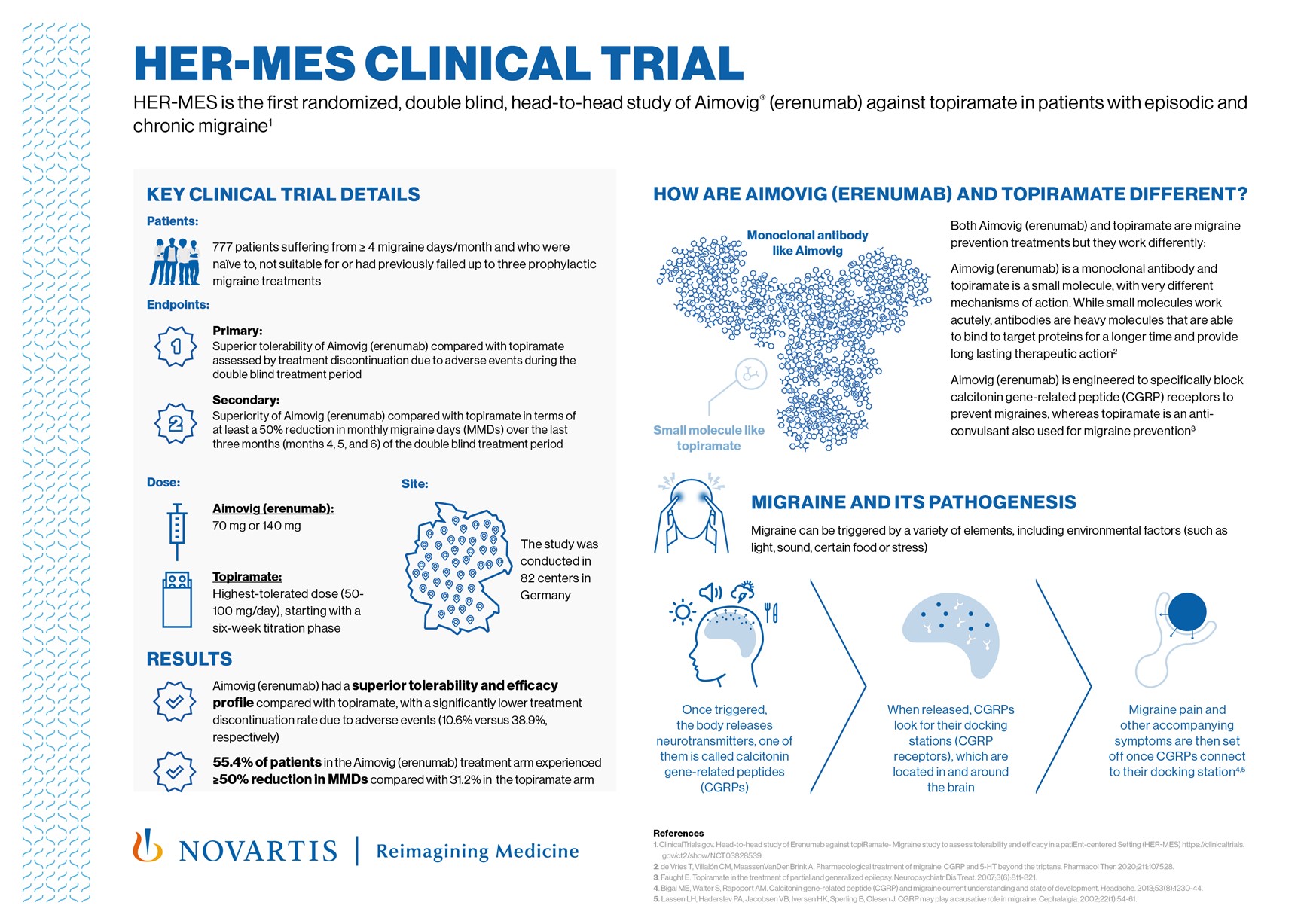
- HER-MES, a Phase IV study, is the first and only randomized, double blind, head-to-head study that compares an antibody targeting the CGRP pathway, Aimovig® (erenumab), with an oral prophylactic migraine treatment (topiramate)1
- Aimovig demonstrated superior tolerability and efficacy against topiramate, with 55.4% of patients in the Aimovig group achieving a reduction in monthly migraine days of at least 50% from baseline compared with 31.2% in the topiramate group1
- Aimovig is the most utilized anti-CGRP treatment, with more than half a million patients prescribed worldwide since launch2
The digital press release with multimedia content can be accessed here:
Basel, November 8, 2021 — Novartis announced today that data from HER-MES has been published in Cephalalgia1. This is the first and only randomized, double blind, double-dummy, active-controlled, parallel-group Phase IV study of Aimovig (erenumab) against topiramate, an anticonvulsant, in patients with episodic and chronic migraine. Results showed that Aimovig had a superior tolerability profile compared with topiramate, with a significantly lower treatment discontinuation rate due to adverse events (10.6% with Aimovig® vs 38.9% with topimarate)1. Aimovig also demonstrated superior efficacy against topiramate, with patients having a significantly higher probability of achieving a clinically meaningful improvement in migraine frequency when they were randomized to Aimovig compared to topiramate (55.4% vs 31.2%)1. The positive outcomes in the Aimovig group translated into a major improvement in quality of life and in functional impairment for the patients.
“HER-MES is the first study that directly compared the therapeutic effect of an antibody and a small molecule in migraine prevention,” said Prof. Uwe Reuter, Managing Medical Director at Charité Universitätsmedizin. “The positive outcomes strengthen the position of erenumab as a safe and effective migraine prevention treatment that can significantly enhance quality of life for migraine patients with an improved dosing regimen.”
“Results of this first and only head-to-head study show superior tolerability and efficacy for Aimovig versus topiramate, further demonstrating the value of Aimovig to patients living with migraine,” said Lykke Hinsch Gylvin, Neuroscience Global Medical Franchise Head, Novartis Pharmaceuticals. “We are proud to continue reimagining migraine care by providing a safe and effective preventive treatment option to patients living with this highly debilitating disease.”
About HER-MES
HER-MES (NCT03828539) is a randomized, double blind, double-dummy, active-controlled, parallel-group Phase IV study to assess the tolerability and efficacy of Aimovig® (erenumab) versus topiramate in a patient-centered setting1. The primary endpoint was treatment discontinuation rate due to adverse events of 70 mg and 140 mg erenumab compared with 50–100 mg/day topiramate during the double blind treatment phase of the study1. The secondary endpoint was efficacy of 70 mg and 140 mg erenumab versus the highest-tolerated dose (50-100 mg daily) of topiramate in terms of at least a 50% reduction in monthly migraine days (MMDs) from baseline in the last three months (Months 4, 5 and 6) of the double blind, 24-week treatment phase1. The HER-MES study enrolled 777 adult patients with episodic or chronic migraine (≥4 migraine days per month) who had not previously received migraine prevention treatment or had failed three previous therapies with propranolol/metoprolol, amitriptyline and flunarizine1. After a two-week screening and four-week baseline phase, patients were randomized 1:1 to erenumab or topiramate. In the double blind, 24-week treatment phase, patients in the erenumab arm received either 70 mg or 140 mg directly after the baseline phase1. An increase in dose from 70 mg to 140 mg was possible at any time during the study. Patients in the topiramate arm were given topiramate at the highest-tolerated dose (50-100 mg), starting with a six-week titration phase1. The study was conducted at 82 study sites in Germany between February 2019 and July 2020.
About Aimovig® (erenumab)
Aimovig is the first European Medicines Agency (EMA), Swissmedic, and U.S. Food and Drug Administration (FDA)-approved migraine prevention treatment designed specifically to block the anti-calcitonin gene-related peptide receptor (CGRP-R), which plays a critical role in migraine. Aimovig has been studied in several large, global, randomized, double blind, placebo-controlled studies to assess its safety and efficacy in migraine prevention. More than 3,000 patients have participated in our overall clinical trial program. This includes over 2,600 patients across the four placebo-controlled, Phase II and Phase III clinical studies as well as participants in further studies such as LIBERTY, a dedicated study in a treatment failure population that is difficult to treat3. The most common side effects in the clinical program to date have been viral upper respiratory tract infection, sinusitis, influenza and back pain. Aimovig is the most utilized anti-CGRP treatment worldwide, with more than 520,000 patients prescribed in the post-trial setting1.
Novartis and Amgen are co-commercializing Aimovig in the US. Amgen has exclusive commercialization rights to the drug in Japan and Novartis has exclusive rights to commercialize in the rest of the world.
About Migraine
Migraine is a distinct neurological disease4. It involves recurrent attacks of moderate-to-severe head pain that is typically pulsating, often unilateral and associated with nausea, vomiting and sensitivity to light, sound and odors5. Migraine is associated with personal pain, disability, reduced quality of life and financial cost to society6. It has a profound and limiting impact on an individual’s ability to carry out everyday tasks. The World Health Organization reported migraine to be one of the top 10 causes of years lived with disability for men and women6,7. It remains under-recognized and under-treated6,8.
Disclaimer
This media update contains forward-looking statements within the meaning of the United States Private Securities Litigation Reform Act of 1995. Forward-looking statements can generally be identified by words such as “potential,” “can,” “will,” “plan,” “may,” “could,” “would,” “expect,” “anticipate,” “look forward,” “believe,” “committed,” “investigational,” “pipeline,” “launch,” or similar terms, or by express or implied discussions regarding potential marketing approvals, new indications or labeling for the investigational or approved products described in this media update, or regarding potential future revenues from such products. You should not place undue reliance on these statements. Such forward-looking statements are based on our current beliefs and expectations regarding future events, and are subject to significant known and unknown risks and uncertainties. Should one or more of these risks or uncertainties materialize, or should underlying assumptions prove incorrect, actual results may vary materially from those set forth in the forward-looking statements. There can be no guarantee that the investigational or approved products described in this media update will be submitted or approved for sale or for any additional indications or labeling in any market, or at any particular time. Nor can there be any guarantee that such products will be commercially successful in the future. In particular, our expectations regarding such products could be affected by, among other things, the uncertainties inherent in research and development, including clinical trial results and additional analysis of existing clinical data; regulatory actions or delays or government regulation generally; global trends toward health care cost containment, including government, payor and general public pricing and reimbursement pressures and requirements for increased pricing transparency; our ability to obtain or maintain proprietary intellectual property protection; the particular prescribing preferences of physicians and patients; general political, economic and business conditions, including the effects of and efforts to mitigate pandemic diseases such as COVID-19; safety, quality, data integrity or manufacturing issues; potential or actual data security and data privacy breaches, or disruptions of our information technology systems, and other risks and factors referred to in Novartis AG’s current Form 20-F on file with the US Securities and Exchange Commission. Novartis is providing the information in this media update as of this date and does not undertake any obligation to update any forward-looking statements contained in this media update as a result of new information, future events or otherwise.
About Novartis
Novartis is reimagining medicine to improve and extend people’s lives. As a leading global medicines company, we use innovative science and digital technologies to create transformative treatments in areas of great medical need. In our quest to find new medicines, we consistently rank among the world’s top companies investing in research and development. Novartis products reach nearly 800 million people globally and we are finding innovative ways to expand access to our latest treatments. About 108,000 people of more than 140 nationalities work at Novartis around the world. Find out more at https://www.novartis.com.
Novartis is on Twitter. Sign up to follow @Novartis at https://twitter.com/novartisnews
For Novartis multimedia content, please visit https://www.novartis.com/news/media-library
For questions about the site or required registration, please contact [email protected]
References
- Reuter U, Ehrlich M, Gendolla A, et al. Erenumab versus topiramate for the prevention of migraine – a randomised, double-blind, active-controlled phase 4 trial. Cephalalgia. Article first published online: November 7, 2021 doi:10.1177/03331024211053571.
- Novartis Financial Results – Q1 2021, April 27th, 2021. Available from: https://www.novartis.com/sites/www.novartis.com/files/2021-04-interim-financial-report-en.pdf [Last accessed: September 2021].
- LIBERTY clinical trial. Available from: https://clinicaltrials.gov/ct2/show/NCT03096834 [Last accessed: September 2021].
- Migraine Research Foundation. Migraine facts. Available from: https://migraineresearchfoundation.org/about-migraine/migraine-facts/ [Last accessed: September 2021].
- National Institute for Neurological Disorders and Stroke. Migraine Information Page. Available from: https://www.ninds.nih.gov/Disorders/All-Disorders/Migraine-Information-Page [Last accessed: September 2021].
- World Health Organization. Headache disorders. Available from: http://www.who.int/mediacentre/factsheets/fs277/en/ [Last accessed: September 2021].
- GBD 2015 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388(1053):1545–1602.
- Diamond S, Bigal ME, Silberstein S, et al. Patterns of diagnosis and acute and preventive treatment for migraine in the United States: Results from the American Migraine Prevalence and Prevention Study. Headache. 2007;47(3):355–363.
# # #
Novartis Media Relations
E-mail: [email protected]
| Amy Wolf Novartis External Communications +41 79 576 0723 (mobile) [email protected] Julie Masow Novartis US External Communications +1 862 579 8456 [email protected] | Meghan O’Donnell Novartis Global Pharma Communications +41 79 797 9102 (mobile) [email protected] |
Novartis Investor Relations
Central investor relations line: +41 61 324 7944
E-mail: [email protected]
| Central | North America | ||
| Samir Shah | +41 61 324 7944 | Sloan Simpson | +1 862 345 4440 |
| Thomas Hungerbuehler | +41 61 324 8425 | Alina Levchuk | +1 862 778 3372 |
| Isabella Zinck | +41 61 324 7188 | Parag Mahanti | +1 973-876-4912 |