Key Releases are ad hoc announcements pursuant to SIX Swiss Exchange Article 53 Listing Rules.
Header
News Archive
News Archive Navigation
icon
News Archive Navigation Language
Language Preferences
Showing 1811 results
June 2016
-
Media Release
Novartis data show more than 50 percent of eligible Ph+ CML patients maintain Treatment-free Remission (TFR) after stopping Tasigna®
In ENESTfreedom, 51.6% of eligible first-line Tasigna patients maintained TFR for 48 weeks after stopping treatment; study did not meet its statistical primary endpoint, specifically the >… -
Story Patient Perspectives

Your Genes, Your Melanoma
Learn about the role of precision oncology in advanced melanoma treatment.
-
In The News
Novartis Access hosts second stakeholder dialogue
The second stakeholder dialogue shared first learnings from the Kenya launch, and to continue the discussion on how to address the burden of NCDs in lower-income countries. -
Media Release
Novartis drug Afinitor® receives EU approval to treat certain types of advanced gastrointestinal (GI) and lung neuroendocrine tumors (NET)
Afinitor fills critical need in EU as first approved therapy for advanced, progressive, nonfunctional lung NET and first oral therapy for this type of GI NET Nonfunctional GI and lung NET are…
May 2016
-
Media Release
Novartis highlights its strong foundation for long-term, sustainable growth at the third Meet Novartis Management event
Outlines actions underway to accelerate launch of Entresto®, including further expansion of US primary care field force, and reinforce strong uptake of Cosentyx® Highlights… -
Media Release
Sandoz' biosimilar rituximab regulatory submission accepted by European Medicines Agency
Sandoz advances biosimilar portfolio with sixth major biosimilar file acceptance in less than one year Sandoz is seeking approval for all indications included in the reference product's label… -
Media Release
Novartis' Entresto® given strong Class I recommendation in both US and EU heart failure guidelines, less than a year after regulatory approvals
US guidelines now recommend Entresto as standard of care for HFrEF as an alternative to ACEs or ARBs; call for doctors to switch patients with mild to moderate symptoms to Entresto[1] Updated… -
Story From Our Labs
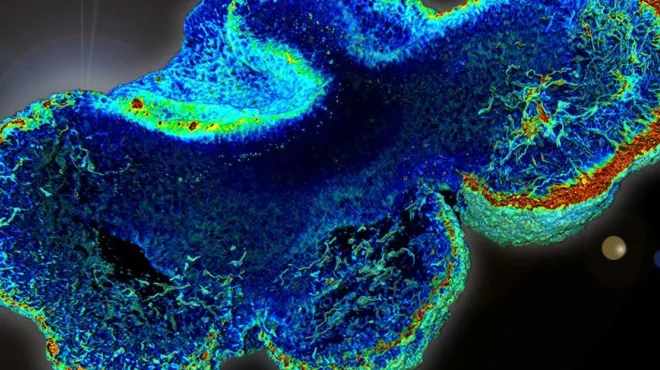
Building brain organoids to shed light on disease
Three-dimensional clusters of human neurons could allow scientists to watch neurological diseases develop in the lab.
-
Story From Our Labs

With a "brain in a dish," researchers take on neurological disorders
Neurological diseases develop out of sight. Novartis neuroscientists are bringing them into the light, using human brain cells grown in the lab.
-
Media Release
Novartis announces investment in FortiHFy clinical program of Entresto® and heart failure
Fortifying Heart Failure clinical evidence and patient quality of life (FortiHFy) is an umbrella clinical program comprising over 40 active or planned trials The global clinical program… -
Media Release
Novartis to present pivotal data in hematologic and solid tumor cancers at 2016 ASCO Annual Meeting
First data from two treatment-free remission (TFR) studies of Ph+ CML patients treated with Tasigna® both in front-line and second-line following Glivec®* First genomic analysis and 3-year… -
Media Release
MONALEESA-2 trial of Novartis' LEE011 (ribociclib) stopped due to positive efficacy results at interim analysis in HR+/HER2- advanced breast cancer
Independent Data Monitoring Committee recommends stopping the trial early as it met the primary endpoint, significantly extending progression-free survival (PFS) compared to letrozole alone, at pre-…
Pagination
- ‹ Previous page
- 1
- …
- 125
- 126
- 127
- 128
- 129
- 130
- 131
- …
- 151
- › Next page