Noise and toxin exposures had damaged the tiny sound-sensing hair cells of his inner ear, leaving him with severe hearing loss. To keep working, he opted for the only available option: cochlear implants, microphones mounted behind the ear that transmit sounds to electrodes implanted in the inner ear, bypassing the hair cells to create an electronic approximation of natural hearing.
For new patients with similar hearing loss, however, an exciting option is on the horizon. In May 2014, an experimental drug from Novartis that could potentially restore lost hearing entered early-phase clinical trials. Rather than providing an electronic solution, the drug appears to unlock the body’s ability to regenerate delicate hair cells and repair the ear’s natural auditory mechanics. Much research remains to be done, but if the experimental drug, known by its research designation CGF166, proves effective and safe in this trial, it could lead to a major breakthrough for the treatment of hearing loss.
“This is a very novel attempt to develop a medication targeted at an important inner ear disease,” says Hinrich Staecker, head and neck surgeon at the University of Kansas Medical Center in the USA and the principal investigator for the trial. “We have a lot of workarounds for hearing loss, but it still takes a tremendous toll on day-to-day life.”
Hearing loss can be frustrating, isolating, and disabling -- suffering that is evident from the reaction Staecker received after the trial’s launch. Following coverage of the clinical study in the magazine New Scientist in April 2014 he received more than 600 emails and dozens of daily phone calls from potential patients.
Where need meets science
Disabling hearing loss affects 360 million individuals globally and about a third of people over the age of 65, according to the World Health Organization. In the US, nearly half of those over age 75 suffer significant hearing loss. Despite this need, people have few options. “There are devices, but no drugs for hearing loss,” says Lloyd Klickstein, head of translational medicine for the New Indications Discovery Unit at the Novartis Institutes for BioMedical Research, who guided the advancement of the new therapy.
This is a very novel attempt to develop a medication targeted at an important inner ear disease.
Hinrich Staecker, head and neck surgeon at University of Kansas Medical Center, US.
In 2009, Klickstein and his team were charged with identifying new opportunities for Novartis, focusing on conditions that are well understood scientifically, yet have few treatment options. Of 6,000 conditions he and his colleagues surveyed, “diseases of hearing and balance bubbled to the top,” he says. The team asked the Novartis Strategic Alliances group to help find partnership opportunities in academia or among biotech companies.
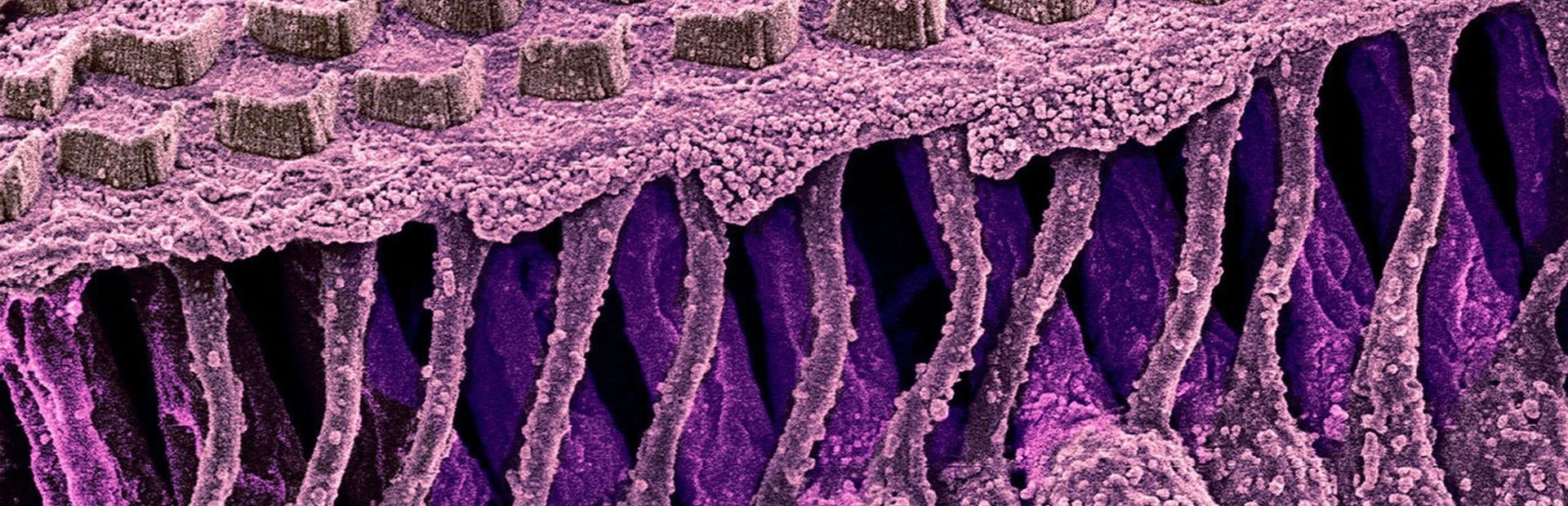
While the medical need was clear, the science also was compelling. In 1999, scientists had singled out atonal, a gene that acts as a “master switch” for turning on the growth of inner ear hair cells, which pick up sound waves and translate them into electrical signals in the brain. Humans are born with hair cells, but the atonal switch flips off at birth. Any subsequent damage to hair cells is permanent. In contrast, birds are able to replace lost hair cells.

Acting on this discovery, a biotech company called GenVec developed a way to flip that switch back on. They used a viral vector, a virus that has been altered with the aim of making it harmless so it can act as a carrying case for atonal. The virus carries the atonal gene into the cells that line the insides of the snail-shaped cochlea, some of which are capable of turning into hair cells. In these cells, atonal appears to cue that transformation. The newly formed hair cells then wire themselves into the part of the brain that processes sound.
Working with academic labs, GenVec researchers showed in 2005 that their vector carrying the mouse version of the gene could restore hearing in deafened rodents. Later, in 2011, scientists showed that this drug also restored balance in rodents.
When Klickstein heard about GenVec’s technology, he asked Susan Stevenson to help. Earlier in her career, Stevenson had spent over a decade working on gene therapy and had experienced the high hopes that it would open the door to powerful new medicines, and the disappointment when it failed to do so. “When I looked at GenVec’s work, I remember thinking—gene therapy has finally found its calling,” Stevenson says.
For Stevenson, the use of gene therapy for hearing loss was a revelation. Instead of trying to achieve life-long gene therapy throughout the body, only the inner ear is exposed to this experimental drug. In addition, the drug needs to be applied only once to kick off regeneration. “It’s not that the technology to do gene therapy has changed, it’s our understanding of where it has the best chance of success,” she says.
Mouse to man
Working with her colleagues from GenVec, Stevenson led the team that took on the responsibility of turning GenVec’s technology into a potential therapy that could be tested in humans. The researchers put the human version of the gene into the viral carrying case and showed that it restores hearing in animals.
There are many causes of hearing loss, including genetics and, in the elderly, a lifetime of accumulated assaults from noise, infections or toxins. For some, however, hearing loss can be traced back to an event, for instance to the use of drugs like kanamycin, an antibiotic so strong that it destroys hair cells. The team used kanamycin to model human hearing loss in mice.

Kanamycin causes more significant inner-ear destruction in mice than in humans, but despite the severity of the damage, the experimental treatment partially restored hearing in the mice and also restored hair cells to 50 percent of normal levels. If humans experience similar results in further research, the new drug could transform the lives of people with hearing loss, reducing social isolation and restoring employment. “There are job modifications you can make for severe to profound hearing loss,” says Staecker. “But wouldn’t it be great to keep working at something you love to do?”
A novel surgical procedure is required to administer the treatment. Surgery to open the inner ear is already performed to insert cochlear implants. But the experimental drug requires something that has never been done before: the injection of fluid into the inner ear, a pea-sized space encased in bone.
Staecker expects to perform the first 45-minute surgical procedure in the summer of 2014 at the University of Kansas Medical Center. The one-shot dose is 20 microliters, or about half a droplet, which matches the volume of a cochlear implant device.
With this experimental drug, Staecker speculates that someone like the firefighter with severe hearing loss might improve to moderate hearing loss, potentially becoming a good candidate for a hearing aid, a solution that could provide more natural sound quality than a cochlear implant. A person with moderate loss might move to mild loss or normal hearing. Evaluation of the first patient will begin in a few months, but trial results are not expected for several years. Only then will researchers have a clear picture of the drug’s efficacy and safety in patients.
Looking further into the future, if the trial results should be positive, this experimental drug might also pave the way for the development of a host of inner-ear medicines to treat hearing and balance troubles.