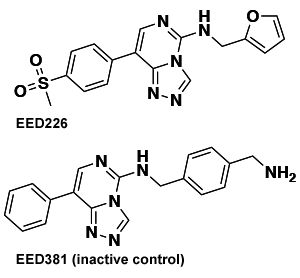
A tumor shares striking similarities with an embryo. It often contains unspecialized cells that are rapidly dividing. In fact, many cancer cells have turned back the developmental clock, spurring growth by adopting the gene expression pattern of embryonic tissue the first few weeks after sperm meets egg. One tool they use to achieve this “primitive” expression pattern is a protein complex called PRC2.
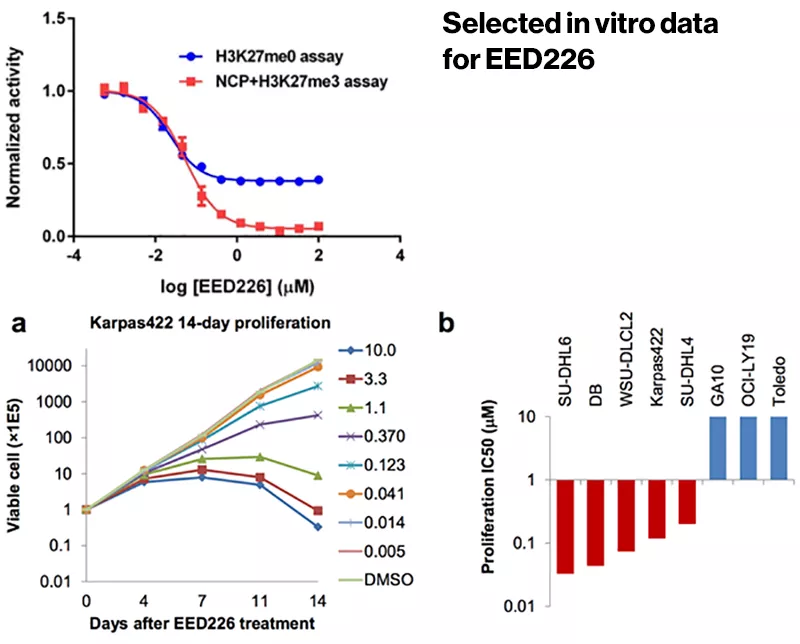
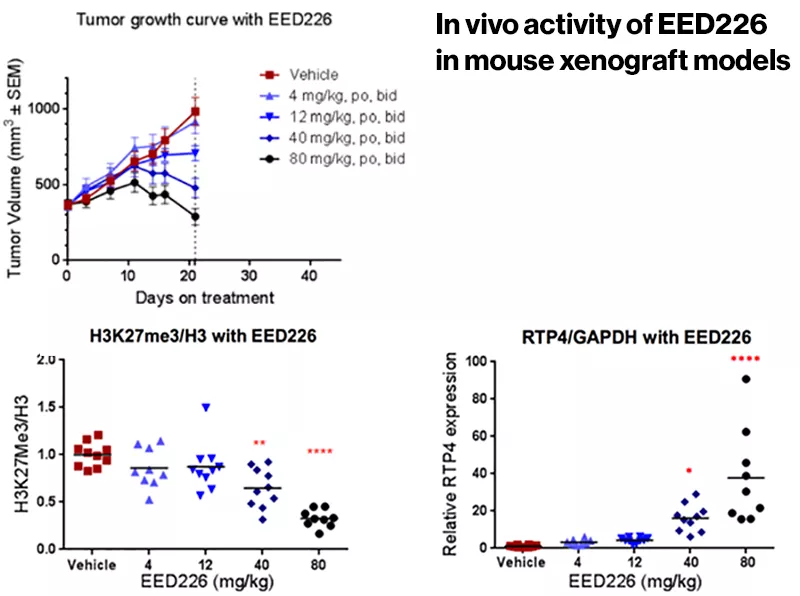
Scientists at the Novartis Institutes for BioMedical Research (NIBR), the global research organization of Novartis, have found a novel way to block the activity of PRC2 and potentially thwart cancer. They have identified a compound that binds EED, a component of PRC2. The compound showed promise in a mouse model of B-cell lymphoma, changing the expression of hundreds of genes and inducing tumor regression, a discovery reported in Nature Chemical Biology. Safety and efficacy remain to be proven in humans.
“This compound reprogrammed the cancer cell genome, changing expression patterns of many genes and suppressing cancer cell growth,” says last author En Li, who heads NIBR’s research site in Shanghai. MAK683—a molecule descended from the compound described in the paper—recently entered Phase I clinical testing.
“We’re one of a handful of companies developing drugs to reverse the sweeping changes in gene expression that occur in cancer cells,” says Li. “This represents a new approach to potentially treating the disease.”
Evolution of epigenetic therapeutics
The approach was born of the understanding that tumor cells may hijack a few key proteins—including those that form PRC2—typically active in the developing embryo to make the changes in gene expression that have been observed. The proteins of interest strategically write and erase marks on our DNA—and on the histone proteins that package our DNA—without changing the underlying genetic code. These “epigenetic” marks help to determine which genes are active and which are silent in a given cell.
In early embryonic cells, PRC2 silences thousands of genes. By the time we reach adulthood, this protein complex is generally silent itself. But some cancers—including many B-cell lymphomas—switch it back on through “activating” mutations in its components, thereby changing the epigenome—which sits above the genome—on a grand scale.
Scientists first realized that epigenetics plays a role in cancer in 1983, but the first epigenetic therapies were approved only recently. These compounds (i.e., DNA demethylating agents and HDAC inhibitors) act broadly on the epigenome, writing or erasing marks on many genes that should be left alone. The next generation of epigenetic therapies—including MAK683, the compound derived from the research in the Nature Chemical Biology paper—promises to be more selective.
“The new compounds are designed to reverse epigenetic changes that help to drive cancer while preserving the rest of the epigenome,” Li says.
The idea is to focus on the proteins driving the epigenetic changes, which explains why researchers have zeroed in on PRC2.
“When I started working in the epigenetics field, PRC2 was literally the most attractive drug target,” says Wei Qi, a biologist and co-first author on the Nature Chemical Biology paper.
Chemical biology in action
Labs around the world were trying to block the portion of PRC2 that marks the epigenome. Instead of focusing on this “active” site, the Novartis team took a different approach, searching for compounds that would inhibit PRC2 through a different mechanism.
The team designed a high throughput chemical screen to identify potential starting points for a drug discovery program. The screen netted molecules that inhibited PRC2 without binding its active site. But the researchers didn’t know how these molecules were working.
“We used chemical biology to tackle this problem,” says Qi. “We took one of the hits from the initial screen and optimized it to probe the biology of PRC2, ultimately revealing a new way to target the protein complex. This required close collaboration between biochemists, biologists, chemists and structural biologists.”
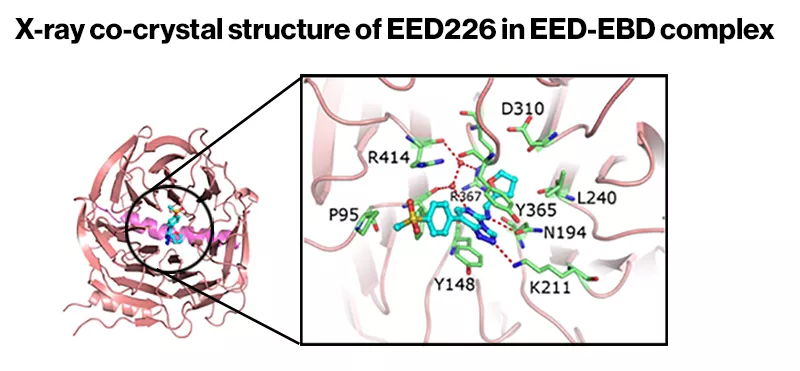
First, the researchers tried to guess where the compound was binding. Ordinarily, PRC2 grabs part of a DNA packaging protein before placing an epigenetic mark. Could the Novartis compound be hitting the portion of PRC2—called EED—that latches onto the DNA packaging protein? Biochemical analyses suggested that this hypothesis was correct.
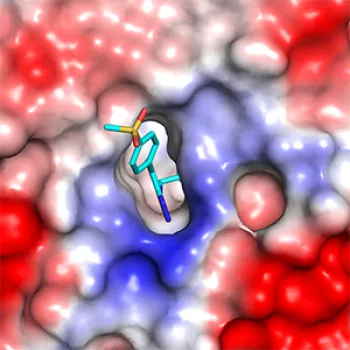
And a crystal structure of the compound bound to EED provided the confirmation that the team needed. It also revealed that the compound works by changing the overall shape of PRC2, inactivating the entire complex.
With a mechanism in hand, the researchers proceeded to animal studies. The compound showed promise in a mouse model of B-cell lymphoma, changing the expression of hundreds of genes and inducing tumor regression.
“Other folks are going after the PRC2 complex, but not in the way that our researchers did,” says Jeffrey Engelman, Head of Oncology at NIBR. “This may ultimately give us a new weapon to deploy against cancer, both as a single agent therapy and in combination with other targeted and immunotherapies. The real impact will be when we determine how to use these combinations for specific cancer populations.”
MAK683 is currently being tested in patients with diffuse large B-cell lymphoma, nasopharyngeal carcinoma and other advanced solid tumors for whom no further effective standard treatment is available. The tool compound from which it’s descended will be supplied to the scientific community to support further exploration of PRC2 biology.