He was visiting his eye doctor for a routine exam 20 years ago when the doctor suddenly exclaimed, "Forty-one? That can't be right. We've got to do that again."
"My eye pressure was astronomical," the 69-year-old management consultant recalls. "And I had no clue."
That's how glaucoma works.
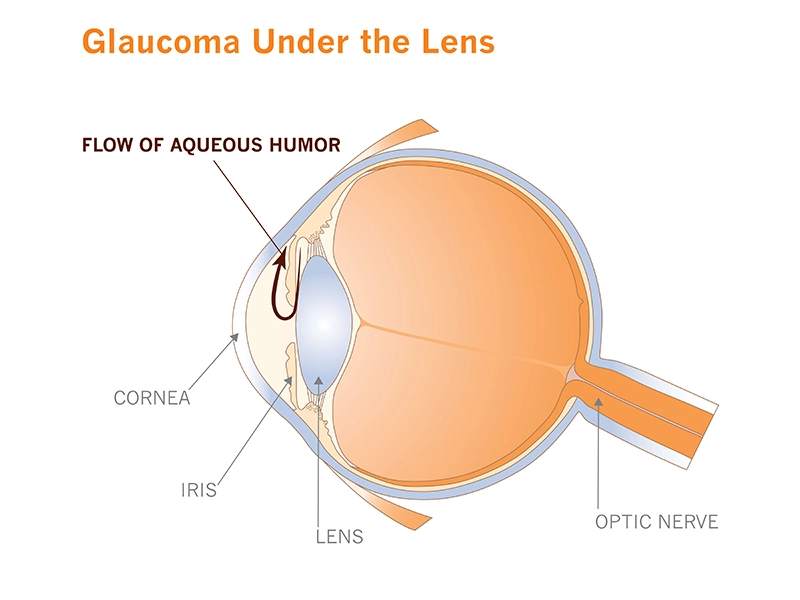
The second leading cause of blindness in the world, it's insidious for its stealth. When it first strikes, it typically doesn't cause pain or noticeable symptoms. Over time it quietly increases the fluid pressure in the eye (what doctors call intraocular pressure), slowly destroying optic nerve cells, narrowing vision to an ever-shrinking tunnel.
"Twenty years ago if I was sitting in a restaurant and a waiter approached me from the left, I could see him coming," says Frohman. "Now if he does that I get surprised because all of a sudden there's a waiter in front of me. Now imagine driving."
Glaucoma ultimately robs some 60 million people worldwide of their sight. Researchers at Novartis want to cut that number dramatically. The company is building a new research team specifically to learn more about what makes glaucoma tick at the cellular level—and to see if any of the 1.6 million compounds on the company's shelves can stop the disease in its tracks.
Lots of options, none of them great
The aim is to improve upon today’s glaucoma medications, which although effective for many patients, have drawbacks and are often not adequate. Most were originally developed to treat other ailments.
The more successful ones have been around for 20 years or more. They are given as eye drops, which can sting or burn and which some patients find unpleasant. Their effect is temporary, so patients have to take them every day, sometimes multiple times. And patients may need to use several at once to achieve the desired result.
"I'm taking two medications now,” Frohman says. “This is the fewest I've ever taken. But at the beginning, my doctor and I experimented with a lot of different medications." Even then, because the medications couldn't keep the pressure in one of his eyes stable, he has undergone four surgeries in the last decade.
The drugs only halt glaucoma’s progression rather than reversing it, meaning that patients don't see any improvement in their vision over time. Thinking that the medications aren't working, they sometimes stop taking them, unwittingly allowing the disease to advance.

"It's the most disheartening thing," says Dennis Rice, an executive director of ophthalmology at the Novartis Institutes for BioMedical Research (NIBR) and leader of the new glaucoma group. "Patients don't see a benefit and stop taking the medication, but when they come to clinic their visual field has larger blind spots. And we have no way to repair that."
Underlying the challenge of treating the disease is a significant knowledge gap. As Douglas Rhee, a glaucoma researcher and chair of ophthalmology and visual sciences at Case Western Reserve University in Ohio in the United States, notes, "Our knowledge of how pressure within the eye is regulated is rudimentary. It's a complex process, and because we don't understand it well, we're not really interrupting the disease process."
A matter of flow
This lack of knowledge prompted Rice to go back to the drawing board in his search for new treatments that will lower pressure in the eye and last longer—and that patients will be more apt to take. He's building a team of molecular and cell biologists within NIBR's Ophthalmology group that is starting from scratch to achieve a singular goal: find out who the thief is by understanding at the cellular and molecular level what precisely goes wrong in the eye that makes the pressure go up.
The magic bullet would be a drug that changes the drainage pathway in such a way the pressure stays low and controlled for many, many months.
Dennis Rice, Executive Director of Ophthalmology at Novartis Institutes for BioMedical Research
For now, Rice and his team have focused on the microscopic plumbing that drains fluid called aqueous humor from the front of the eye. Together, they're creating a set of laboratory tools to study the tissues that make up the “micro-pipes” and to learn more about how they sense and react to pressure in the eye.
"We really have limited knowledge about the molecular make-up of cells that control the plumbing,” Rice says. “In particular, how do these cells respond to different stimuli that might impact pressure regulation?”
Plumbing inspection
While the significance of these changes is not clear, they form a starting point that Rice, Chen, and their colleagues are using to build a new understanding of glaucoma biology.According to Amy Chen, an investigator on Rice's team, the pipes change in ways that aren't fully understood in glaucoma. For instance, their inner linings have fewer cells than normal. And they're not as soft or leaky as they should be.
Using tissue donated by deceased patients to eye banks, Chen’s group is modeling the eye's plumbing in the laboratory—constructing both healthy and diseased versions of the drainage system. Her group will study how cells in the system respond to changes in pressure. The team will eventually use these models to search for new glaucoma drugs. The plan is to expose the diseased version of the drainage system to different molecules, and then determine which of these “treatments” coax sick cells to behave like healthy ones.
Rice has a clear vision for his team's work: produce a drug that not only reduces pressure, but remodels the eye's plumbing, undoing the changes that keep the pressure high. And one that does so for a long time, making life easier for patients.
"The magic bullet," Rice says, "would be a drug that changes the drainage pathway in such a way that the pressure stays low and controlled for many, many months. Long enough that an ophthalmologist doesn't have to worry, 'Is my patient taking the medication? Is the pressure under control?'"
That's a drug Frohman and patients like him would also be happy to see.
This story was updated in March 2019 to reflect business changes at Novartis.