Most children and young adults with acute lymphoblastic leukemia (ALL) who received a Novartis T-cell immunotherapy in two clinical trials responded to the treatment1. Scientists typically didn’t find any molecular traces of the aggressive blood cancer after the treatment, tisagenlecleucel, a form of chimeric antigen receptor T-cell (CAR-T) therapy in which the patient’s own T-cells are genetically re-engineered to wipe out their tumor cells.
Unfortunately, the disease came back after approximately three to 10 months in about a third of these cases, for reasons that were not well understood.
Now scientists at the Novartis Institutes for BioMedical Research (NIBR), together with colleagues at 11 other institutions, have performed a wide-ranging genomic analysis that explains how ALL evolved resistance to tisagenlecleucel in some young patients in the two clinical studies. Reported in Nature Medicine, the study also offers clues about how future combination treatments might help such patients. The work is one part of a large research effort at Novartis to create next-generation CAR-T cell therapies.
This research initiative builds on significant advances in treating young patients with ALL. “We're getting complete remissions within a month’s time among patients in this patient cohort,” says Jennifer Brogdon, Director of the NIBR Cell Therapy Lab. “It’s the immune system doing what it does best, which is quickly recognizing and removing dangerous threats from the body to protect it. But, in fact, tumor cells still have the ability to evolve and acquire resistance to treatment.”
Digging deep to understand relapse
The researchers followed up on two trials that had tested the efficacy and safety of the CAR-T therapy tisagenlecleucel in the treatment of ALL. This drug is designed to target CD19, a protein found on the surface of B immune cells, which turn cancerous in ALL.
Among the 104 patients in the two trials, 32 eventually relapsed. The NIBR scientists studied samples from 12 of the relapsed patients whose tumor cells no longer showed any signs of the CD19 protein. They sequenced tumor DNA to find genetic mutations in the tumors, and they sequenced RNA expression to examine the effects of these mutations on the proteins the tumors produced.
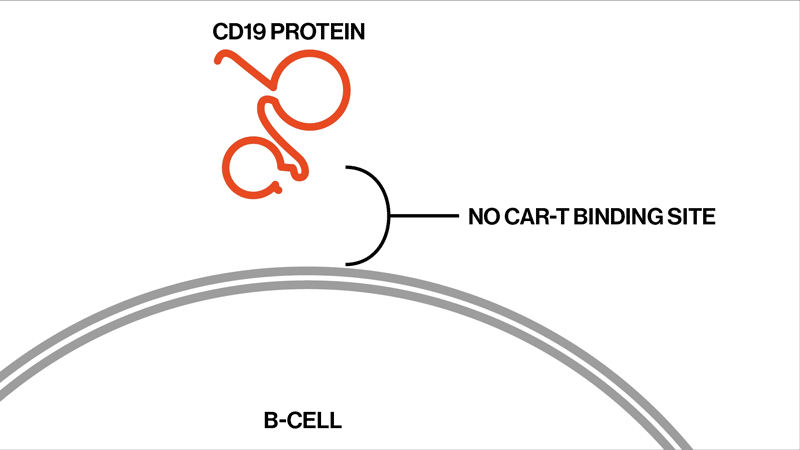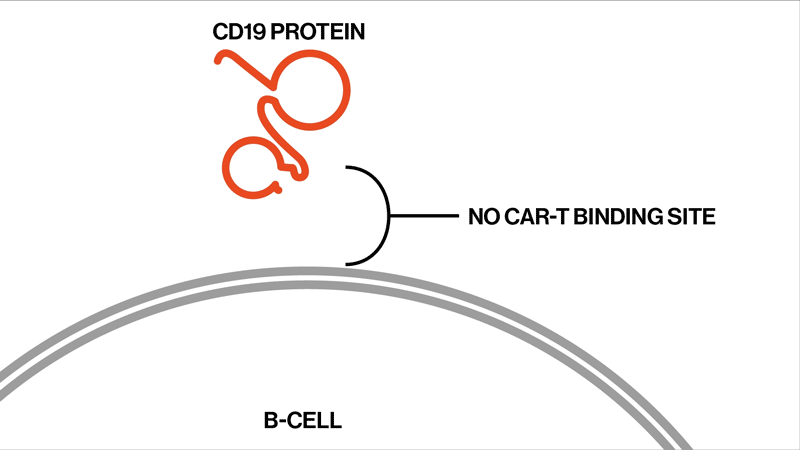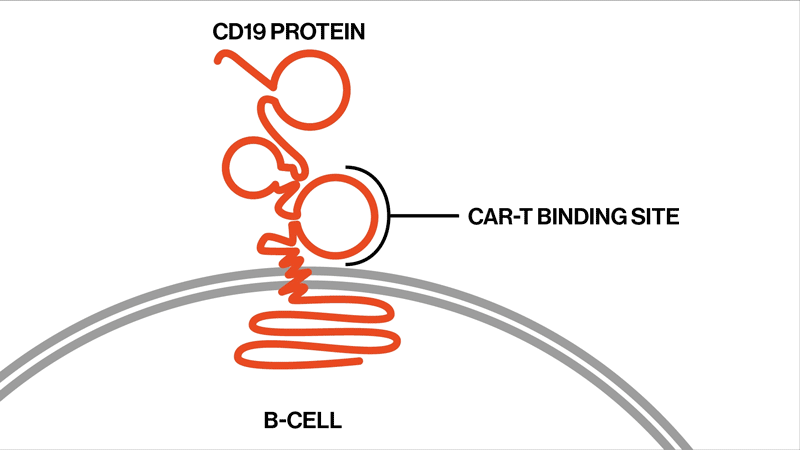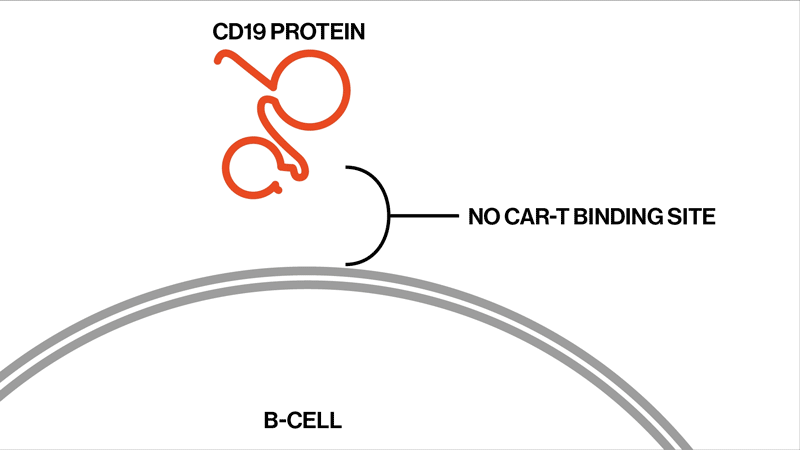
Each patient showed different mutations in CD19, and some patients exhibited a strikingly high number of mutations. “We found that these patients had rapidly acquired genetic mutations in CD19 that would explain why they were no longer expressing the protein on the cell surface,” says Elena Orlando, lead author on the paper and an investigator in NIBR’s Next-Generation Diagnostics group.
Mutations happen by chance among the hundreds of millions of tumor cells, and if a mutation gives a cell a survival advantage, the cell can proliferate extremely quickly. For instance, some of these relapsing patients were monitored each month after initially responding to the therapy, and the percentage of cells with CD19 mutations went from zero to 100% within a month.
“It’s amazing to watch how rapidly these tumors can evolve,” says Wendy Winckler, Executive Director of Next-Generation Diagnostics at NIBR.
The study settles the question about whether these relapsed patients whose tumor cells no longer show signs of the CD19 protein might benefit from another round of tisagenlecleucel, Orlando says. They won’t benefit, because the genetic mutations have created versions of the CD19 protein that can’t be targeted by the therapy. The proteins are too damaged to present themselves on the surface of the tumor cells.
Instead, researchers will look at drug combination strategies to treat these patients, searching for another protein that is particularly likely to appear on the surface of B-cells and not so much on other cells. One likely suspect is a protein known as CD22, and a number of companies are now testing treatments that combine CAR-T for both CD19 and CD22.
Bringing discovery tools to trials
These findings suggest that there is much that Novartis researchers can learn from deep analysis of patients’ tumor samples before treatment and after response or relapse. “We’re taking a deep dive into the patient samples and seeing what we can discover to explain what happens after the CAR-T infusion,” says Brogdon. “We’re trying to uncover insights that will help the field move more quickly toward the next generation of CAR-T therapies and help more patients, which is our ultimate goal.”
NIBR formed the Next-Generation Diagnostics group about five years ago to support such efforts by applying genomics and other cutting-edge discovery technologies to clinical trials.
“We’re working across Novartis oncology trials, applying similar research methods to the unique questions that come up in each of those therapies,” Winckler says. “There’s a big opportunity for a group like ours that uses these comprehensive discovery tools to look outside the box to understand why patients respond or don’t respond.”
Main image: Courtesy Mark Mazaitis
Research provides new insights into CAR-T therapy and #bloodcancer.