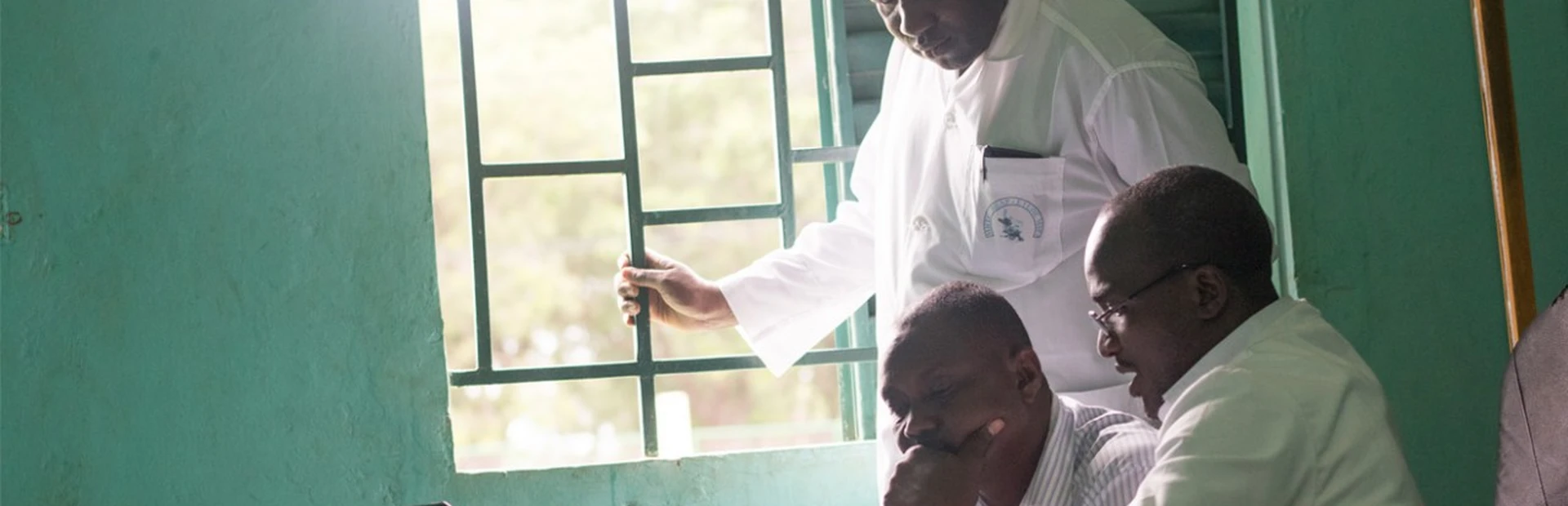
Dr. Bakary Fofana and two colleagues sit at a desk in a health clinic and research lab in southern Mali, writing an email to their collaborators in the United States. Two pre-labeled test tubes for patient blood samples broke during the three-week transit from the US to Mali.
Dr. Fofana has plenty of extra test tubes lying around, but the broken tubes are unique to the clinical trial of an experimental new malaria treatment he is helping conduct. They arrive from the US labeled and numbered, pre-registered in an online database.
Until Dr. Fofana agrees on a solution with the lab in the US, researchers in Mali can’t admit any new malaria patients into the trial. And with eligible patients already at a premium, the team is anxious.
We’ve had to go further and further into the bush to be sure we’re doing the study in areas where the patients are.
David Hughes, Senior Global Program Head, Novartis
Broken test tubes are just one of the many logistical challenges researchers must overcome as they test an experimental new treatment called KAF156 in 16 mostly rural locations across Africa and Asia – where people continue to cope with the often deadly impact of the malaria parasite. Hurdles range from shipping advanced lab equipment to remote areas in multiple countries and training local staff on its use, to ensuring patients follow the strict medical monitoring required to measure the experimental treatment’s performance.
And as malaria prevention programs have become more effective, major cities have seen a decline in malaria incidents, prompting researchers to go to rural areas where the disease still flourishes.

“We’ve had to go further and further into the bush to be sure we’re doing the study in areas where the patients are,” says David Hughes, a Senior Global Program Head at Novartis who is overseeing the KAF156 trial. “And you have to bring all the hardware and software with you.”
Drug-resistant parasites
There is considerable urgency to the research. Nearly half of the world’s population is at risk for catching malaria. And in Asia, the parasite that causes malaria is starting to exhibit early signs of resistance to older drugs used to treat the disease. While the number of cases of malaria worldwide has dropped more than 50% in the past 12 years, an estimated 450 000 people still die annually from the disease – most of them children. Now, the pressure is on to find a new drug combination that is effective against the drug-resistant form of the parasite before it becomes more widespread and starts to undo the progress that’s been made.

KAF156 is from a new class of drugs called imidazolopiperazines that attack the parasite in multiple stages of its reproduction. Novartis is developing KAF156 with scientific and financial support from the Medicines for Malaria Venture (in collaboration with the Bill & Melinda Gates Foundation).
Shipping blood samples
Dr. Fofana – Fofa, as he’s affectionately known by his colleagues – works with his team in a rural part of the Sikasso region that is a five-hour drive from Mali’s capital, Bamako. He is a principal investigator for the trial of KAF156, and the Sikasso clinic is the first site to begin testing the treatment. The clinic, which opened in 2002, has attracted a strong team of researchers and doctors with experience adhering to the rigid protocols of drug trials.

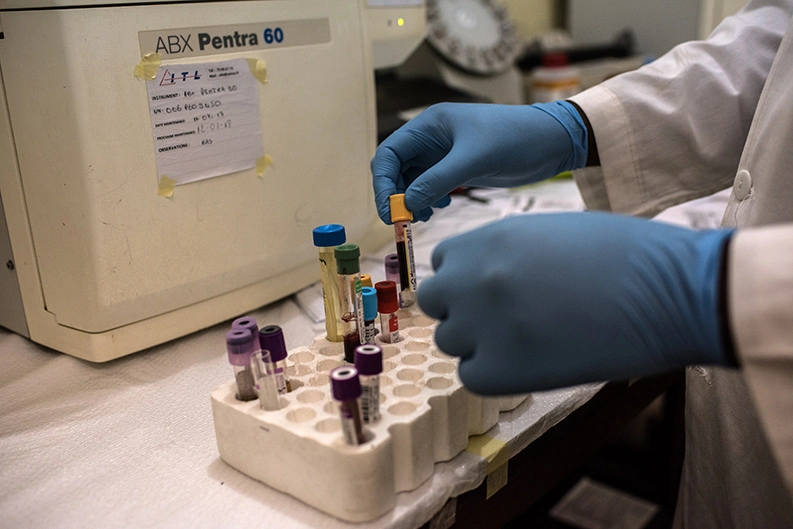
This past August, 12-year-old Allassane Traore sat in the clinic hooked up to a 12-lead electrocardiograph machine that instantly sent the pattern of his heartbeat to an online database. A technician took a sample of his blood down the hall to a lab for analysis.

But some patient blood samples must still be sent to a laboratory with more advanced capabilities. Finding a company capable of handling the shipment of samples to a lab in France presents the team with another challenge. In addition to maintaining strict temperature control the whole way, the shipper must ensure a documented chain of ownership all the way to the lab in accordance with good clinical practice standards. A shipment of blood samples recently waited 10 days in Sikasso until a courier with the necessary capabilities picked it up.
Follow-up exams for patients
Local economic and social factors also complicate matters for the team in Mali. The KAF156 trial requires patients to stay at the clinic for observation for four days so doctors can monitor the effects of treatment. Then they must return once a week for six weeks for follow-up blood tests.
Such a major time commitment is not always feasible for people in the area. For instance, the new Trans-West African Coastal Highway brought new employment opportunities to Sikasso, and many adults in the transportation sector travel for work – making follow-up appointments impossible. That shrinks the pool of potential patients for the trial.

Many of the participants in this trial are young children. Twelve-year-old Adiarra Traore says her four-day stay at the clinic “wasn’t so bad. I got to sleep and eat.” But even having a child be away for four days can be a burden on the family. In Sikasso’s villages, where even the youngest members of the family contribute, such an absence isn’t always an option.

Once patients have been treated and are feeling better, they are sometimes reluctant to return to the clinic for the necessary checkups. To ensure patients come for their weekly follow-up appointments, Dr. Fofana has enlisted the assistance of the village chief’s council of elders, who help track down patients and encourage them to go.
Despite the obstacles, Dr. Fofana and the other researchers in Sikasso are motivated by a sense of urgency. They all believe it’s only a matter of time before drug-resistant strains of the malaria parasite begin to show up in significant numbers in Africa. The only question for them is whether their efforts will help find a new drug first.
In #Mali, scientists are going to where the patients are in a bid to find a treatment for drug-resistant #malaria.