Cancer cells are escape artists by nature. They can dodge drugs designed to cripple them due to one of their defining characteristics: their genetic makeup changes rapidly. Cancer cells are constantly evolving, and, given the right mutation, they’re able to evade treatment.
Scientists are working to block their ability to escape by applying more than one compound to tumors at a time. New combinations may thwart the evolution of drug resistance and may one day achieve a cure. Lab data published this week in Nature support this strategy for chronic myeloid leukemia (CML), a cancer of the blood and bone marrow.
Working with mice with an aggressive form of CML, Novartis scientists tested a carefully designed combination of two molecules—a drug already approved for use in cancer patients, plus an investigational one called ABL001. The combination mounts a two-pronged attack on an abnormal protein that’s found in the cancer cells. In response to the investigational treatment, the tumor cells disappeared from the mice and never returned. It remains to be seen whether such a combination could be safe and effective in humans.
“In addition to quickly eradicating tumors, the combination appeared to keep them from returning, even after treatment was stopped,” says Andrew Wylie, an oncology researcher at the Novartis Institutes for BioMedical Research (NIBR) who led the project.
In contrast, mice that received either molecule individually displayed a transient response to treatment. Their disease came roaring back after initially regressing.
The experiment is part of a broader effort at Novartis to help CML patients. Drugs called tyrosine kinase inhibitors (TKIs) have dramatically improved the prognosis for the disease, but to control it, most patients must take medication every day for the rest of their lives. Researchers are working toward a day when CML patients can safely stop taking pills without regressing.

Clinical trials are underway to determine if patients that have responded to TKI therapy can discontinue their treatment under carefully controlled conditions. But some patients—especially patients with a prior history of resistance to TKI drugs—seem to harbor a small reservoir of tumor cells that multiply when treatment ceases. And patients who have not achieved a deep molecular response do not qualify for treatment-free remission trials in the first place, even if their disease is well-controlled clinically. A deep molecular response means that the level of the abnormal protein targeted by the TKIs is extremely low as measured by the most sensitive technology available.
“We need to add new tools to our therapeutic arsenal to help as many patients as possible achieve deep molecular responses with the hope of one day potentially achieving a cure,” says Renaud Capdeville, Global Program Head for Hematologic Malignancies in Global Drug Development at Novartis.
ABL001 was born of this goal.
Birth of a molecule
“We asked, ‘For patients with CML, how do we design a drug that could potentially achieve a cure?’” says Wylie.
TKIs currently approved for the disease share the same drug target: an over-active protein called BCR-ABL that’s present in the cancer cells of most CML patients. In fact, the existing compounds all hit the same general region of the abnormal protein, blocking the site that it uses to signal cells to grow and divide.
When drug resistance arises in CML patients, it almost always occurs through a mutation in this region of BCR-ABL. Cancer cells are dependent on the protein for survival and reactivate it in a predictable fashion after a compound shuts it down.
The Novartis researchers wondered if they could create a new molecule that would target a different region of BCR-ABL. They reasoned that a multipronged attack on the key protein might thwart the evolution of resistance and eviscerate the enemy for good.
“If an existing TKI plus this hypothetical molecule were simultaneously bound to BCR-ABL, the cancer cell would need to simultaneously harbor resistance mutations in two different regions of the protein to evade treatment,” says Wylie. “The probability of this occurring spontaneously is very unlikely.”
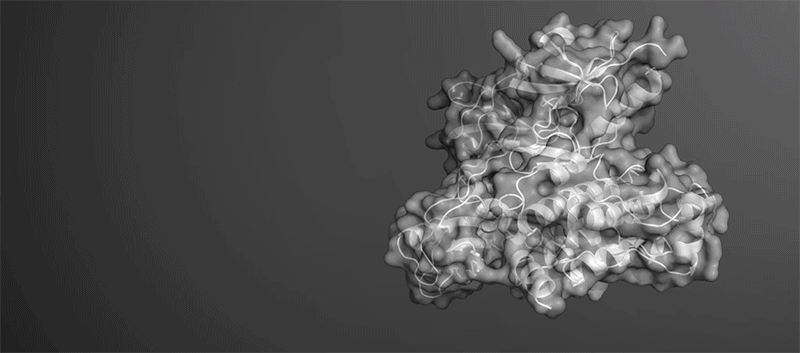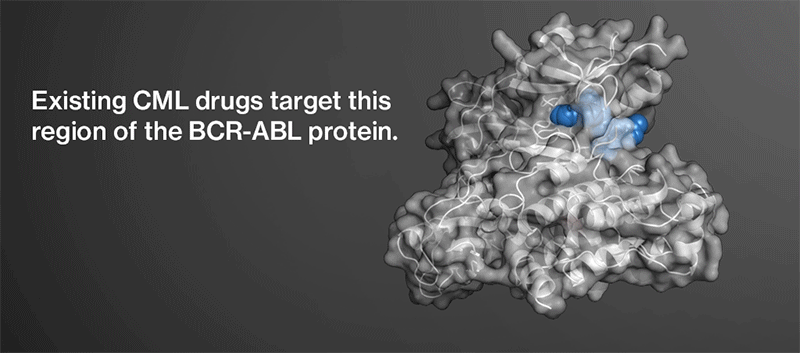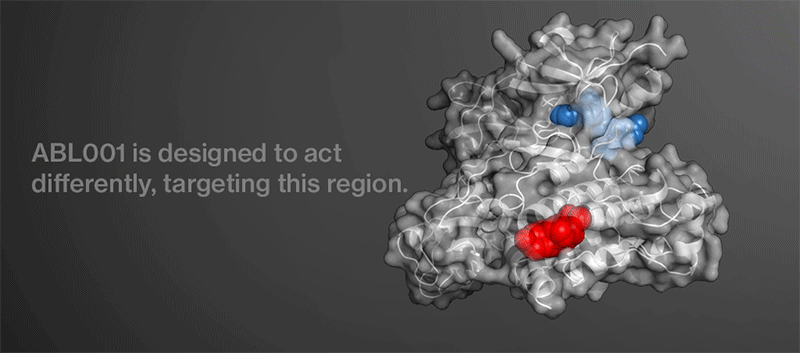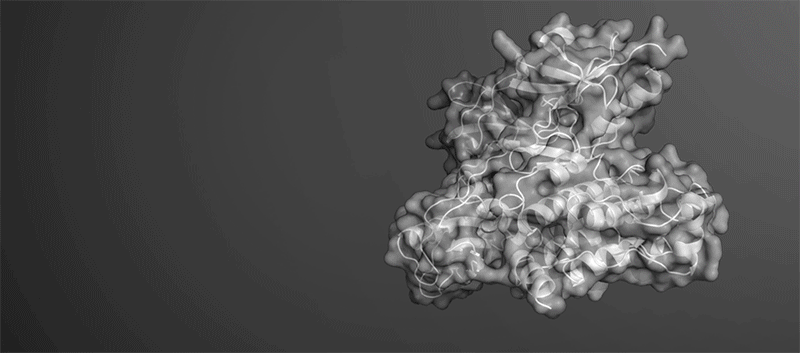
Chemists got to work trying to create the hypothetical molecule. They had a lead from earlier work carried out by researchers at the Genomics Institute of the Novartis Research Foundation. After performing multiple chemical screens and experiments, the team identified a precursor to ABL001. They discovered an investigational compound that may shut down BCR-ABL in a new way. Structural analysis revealed that the compound may bind to a region of BCR-ABL that controls the protein’s overall shape rather than the region that it uses to signal.
“This wasn’t an easy site to target,” says Wylie. “Pockets as greasy as this one present enormous challenges for the chemists to develop molecules with the necessary drug-like properties.”
But the team persevered. They refined the structure of their compound to make it more drug-like and ultimately created the investigational compound ABL001.
Put to the test
In the pivotal experiment highlighted in Nature, the team tested ABL001 on mice with a particularly aggressive type of CML. The tumor cells in the animals are known to quickly develop drug resistance.
The team initially administered ABL001 by itself to one group of mice. They also administered a TKI inhibitor that’s already approved for use in CML by itself to a second group of mice. The tumor cells rapidly regressed in all of the mice, but eventually developed resistance. The mice relapsed.
At this point, the scientists switched the treatment for each group of mice, giving ABL001 to the second group and the approved TKI inhibitor to the first. Again, the disease regressed before surging back.
This cycle of regression and relapse was broken in a third group of mice. When the team administered ABL001 in combination with the approved TKI inhibitor, the tumor cells disappeared. The team monitored the mice for 3 months after stopping the treatment, which was administered for 10 weeks. The tumors did not return during this 3 month period. Three months is a long time to wait for an aggressive form of CML to progress. There was no reservoir of cancer cells waiting for the chance to mutate and multiply. It appeared to have been eliminated.
“Time is your enemy when it comes to resistance,” says Gary Vanasse, a hematologist and ABL001 Program Lead in Translational Clinical Oncology at NIBR. “We hit the cancer cells hard and fast while potentially blocking their escape routes.”
The team hopes the approach can eventually be applied to newly diagnosed CML patients. The idea is to destroy more tumor cells faster.
But first the molecule will be studied in patients who are relapsed, refractory or intolerant to existing TKIs. These patients need new treatment options as soon as possible. Vanasse and his team designed the first clinical study of ABL001, which is being tested in a Phase I trial as a single agent and in combination with several TKIs.
Main image by PJ Kaszas.
Two-compound combo appears to rid mice of chronic myeloid leukemia.