In case you missed it, the Three-Eyed Raven is a character on the TV series “Game of Thrones” that can see the past, present and future. It also happens to be the pet name of a microscope at the Novartis Institutes for BioMedical Research (NIBR). A super-resolution microscope.
One of its jobs is to help Novartis drug hunters understand the past, present and future of brain cells destroyed by Alzheimer’s disease.
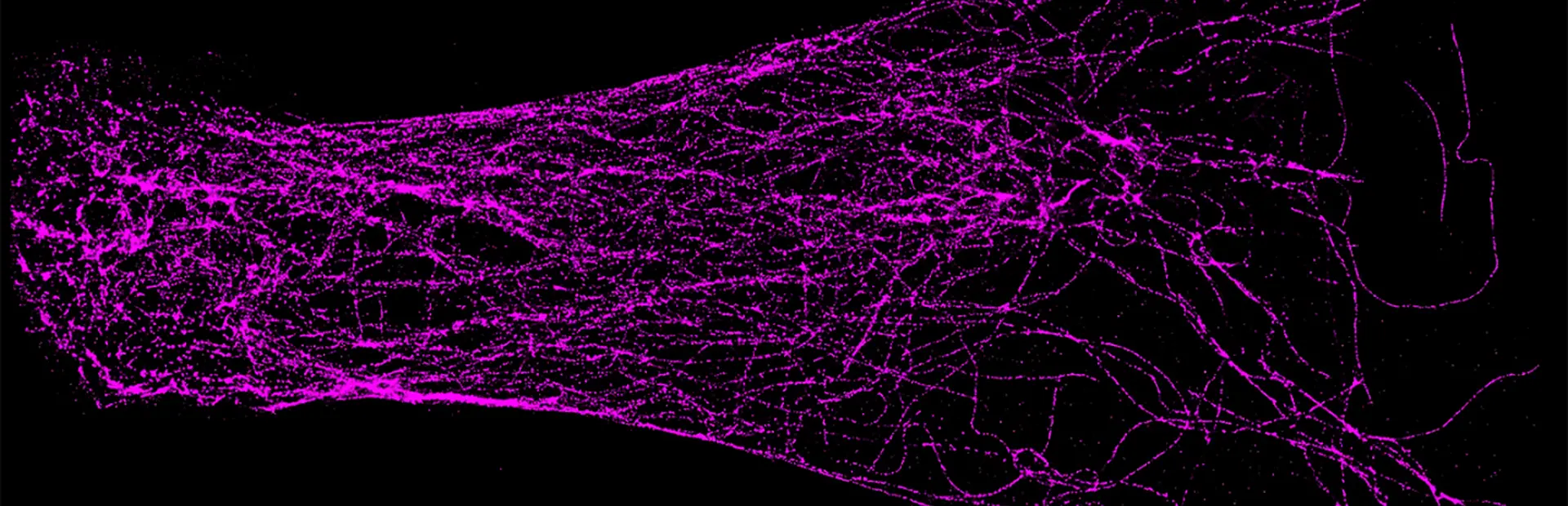
Super-resolution microscopy does exactly what the name implies: It gives super powers to light microscopes, which enable scientists to see small things such as cells. Through super-resolution microscopy, drug hunters can see individual molecules deep inside cells. The technology could help them zoom in on more effective treatments for diseases like Alzheimer’s, which is poorly understood and currently untreatable. Because the technology isn’t ready-made for drug hunting, Novartis is also investing in data scientists who are able to help researchers use the technology in their hunts.
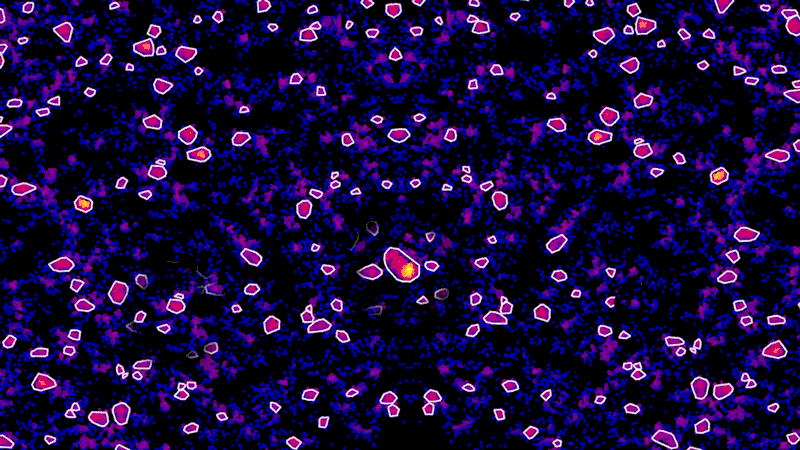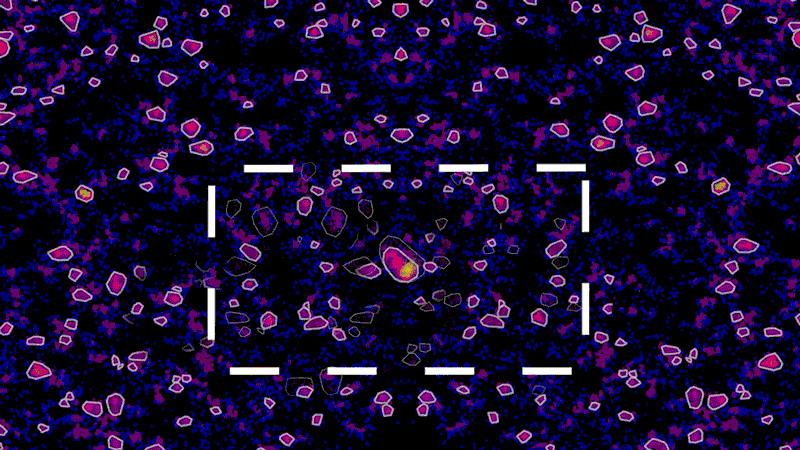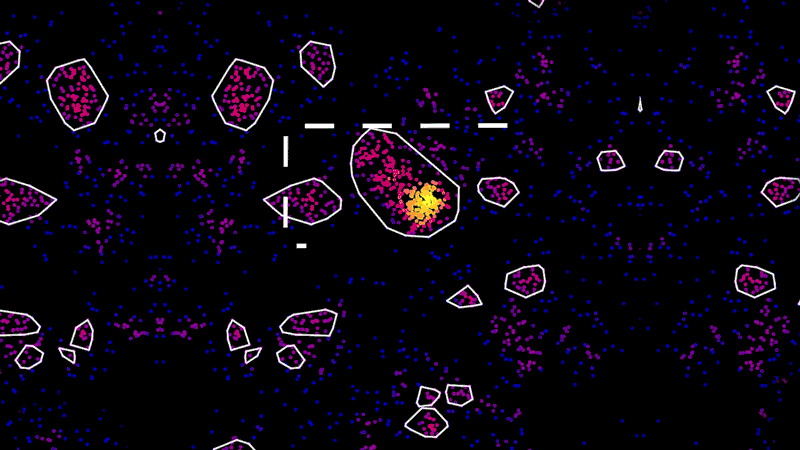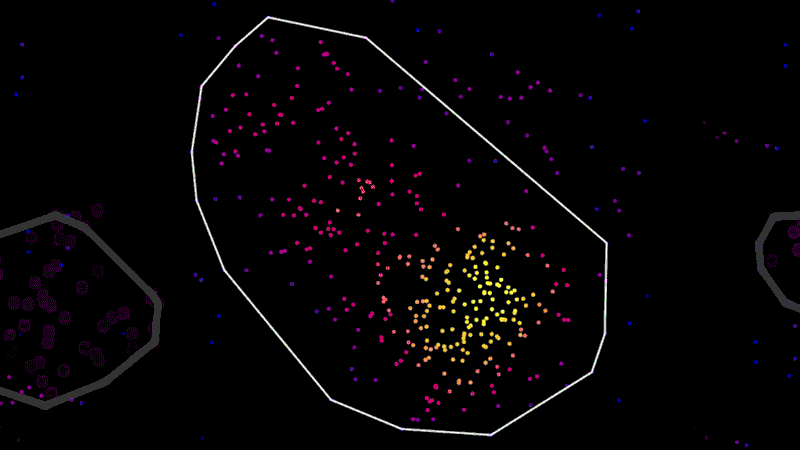
As an example, using super-resolution microscopy, researchers can see how toxic proteins associated with Alzheimer’s disease accumulate, one molecule at a time, from the very first sticky interaction to the eventual formation of globs that ultimately are linked to the degeneration and death of neurons. NIBR scientists are using the technology to investigate the accumulation of a toxic protein called tau.
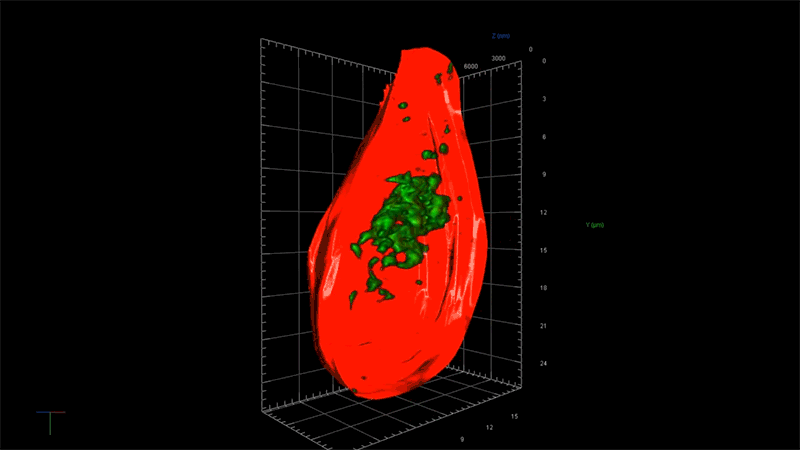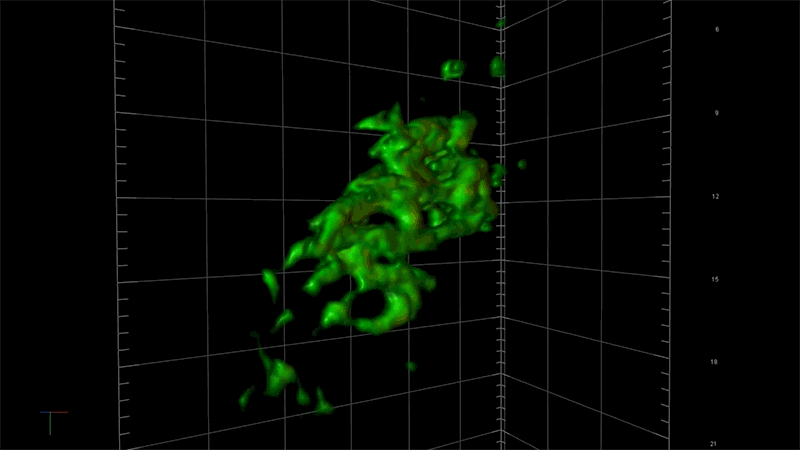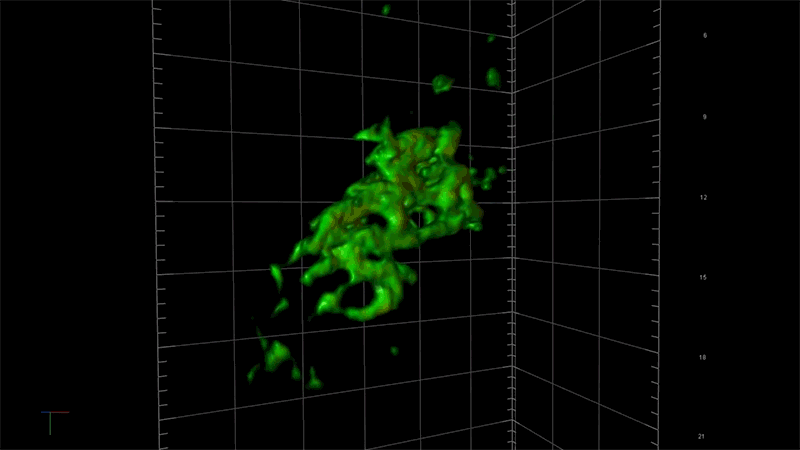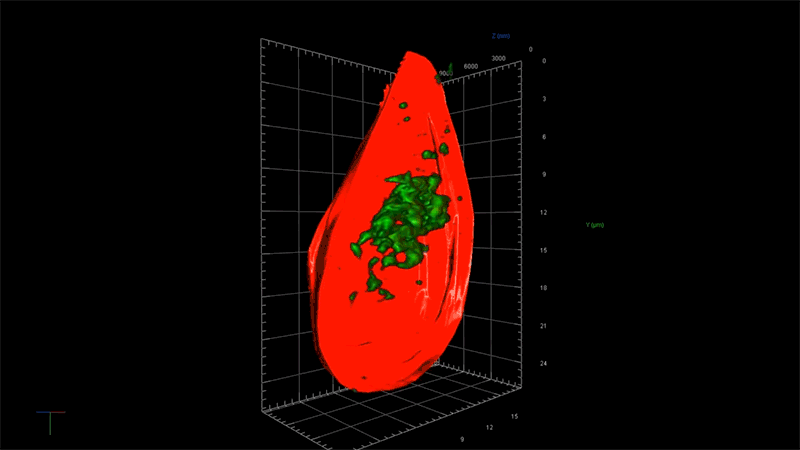
“With this technology, if we can measure the very earliest step of tau aggregation, we can ask what influences it. What genes? What pathways?” says Fiona Elwood, who leads the neurodegeneration group in NIBR Neuroscience. “With regular microscopy, we can’t see anything until we’ve already got big clumps.”
Deep views inside cells with super-resolution microscopy
Normally, a microscope uses light to capture images. The detail of the image is limited by properties of light itself. There are certain things that are too small to see – such as individual protein molecules inside a cell – because light waves are just too big to illuminate them.
To get around this limit, one form of super-resolution microscopy uses laser technology and fluorescent markers that attach to specific proteins in cells to light up one molecule at a time in a way that enables the light microscope to detect it. A series of flashes of a laser enables the microscope to capture each individual protein in a cell and pinpoint its location. It can then be painted on an image that emerges as a series of specks.
“It’s like those cool artists, the pointillists. From a distance you see a picture, but up close it’s all dots,” says Ned Kirkpatrick, Head of Microscopy at NIBR.
The microscope, however, isn’t the pointillist. It merely collects the location data. The real artist is the data scientist, whose role is to build tools that turn that data into an image that is physically accurate and biologically meaningful.

“These new microscopy techniques generate beautiful data. But it’s terabytes and terabytes of raw data,” says Zach Barry, a Novartis data scientist working with the team to apply super-resolution microscopy to neuroscience. “It’s not possible for humans to go through it by eye and answer the questions our scientists want to answer, such as, did this compound work better than that one?”