Your body has a built-in sensor that self-assembles inside cells in response to a threat. This danger-sensing system is a large piece of cellular machinery, but until 20 years ago, no one knew it existed.
Today, however, scientists have learned that this system can get stuck in the “on” position. When it does, it could fuel the flames of diseases such as cancer.
What if we could inhibit an overactive danger-sensing system? Medicines that target this system have the potential to help stop damaging inflammation where it starts. We’re trying to find out if they can offer doctors a new way to help patients with cancer and other diseases such as osteoarthritis and Alzheimer’s disease.
A 150-year-old idea
Rudolf Virchow was the modern version of an early adopter. He loved tech – and the tech of his era was the microscope.
Through its lenses, he observed tissues at the cellular level, a standard procedure now, but controversial for the late 1800s. The study of disease still relied on naked-eye observations of tissues rather than deeper cellular views, even though the idea of microscopic entities called “cells” had been around for centuries.
Virchow used his microscopic observations to change the course of biology by linking cells with disease. In the field of cancer, his ideas about cells suggested, for the first time, that cancer is a collection of healthy cells gone rogue.
Virchow did not stop there. Microscopy revealed another idea – one that would remain unproven and mysterious for over a century: Virchow saw signs that chronic inflammation might fuel the flames of cancer.
Fever findings

In the 1970s, a scientist named Charles Dinarello at the University of Colorado, in the US, was trying to understand a different kind of fire in the body: fevers. He discovered a fever-producing molecule and called it a pyrogen – a fire starter.
His discovery led to an understanding that the body has danger sensors. In response to danger, a cascade of signals – like fires atop mountains calling distant villages to aid – trigger the immune system to respond.
One of the signals in this complex biochemical cascade is called IL-1 beta, which alerts key cells in the immune system to launch an inflammatory response. This kind of inflammation has familiar effects, such as having a fever during a viral infection or joint pain after an injury.
Scientists were finally able to name the inflammatory signals that Virchow had imagined a century ago. Yet the source of these signals remained unknown.
Clues from the human genome

Certain forms of fever were still puzzling scientists in 2001. Researchers from the National Institutes of Health, in the US, were trying to understand why some people with a rare fever syndrome experience near-constant fevers.
With help from advances such as the mapping of the human genetic code, they found a clue. People with these fever syndromes share a mutation in the gene that codes for a danger-sensing protein.
The human body has many of these danger sensors, most of which notice bacteria and viruses. When this particular protein, called NLRP3, senses danger, it curls up and self-assembles like a swarm of microscopic robots into a molecular machine called an inflammasome. Its job is to send out flares – such as IL-1 beta.
Fever syndromes occur when this machine gets stuck in the “on” position, for instance due to a mutation. Constant triggering of the inflammasome results in chronic inflammation and nonstop fevers.
A new view of cancer
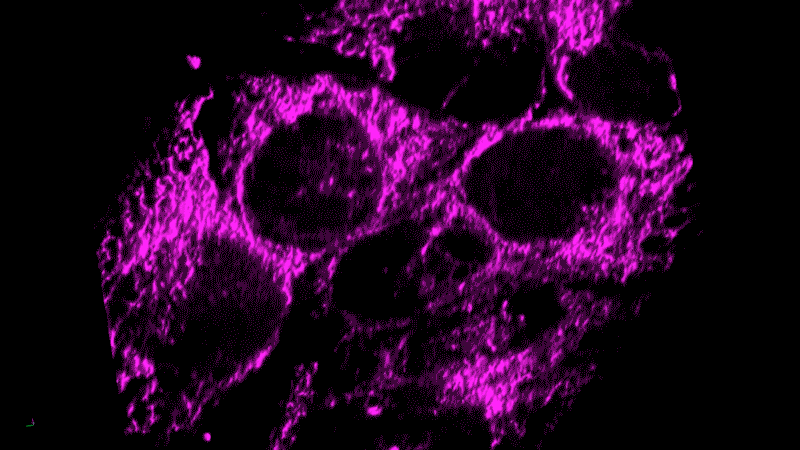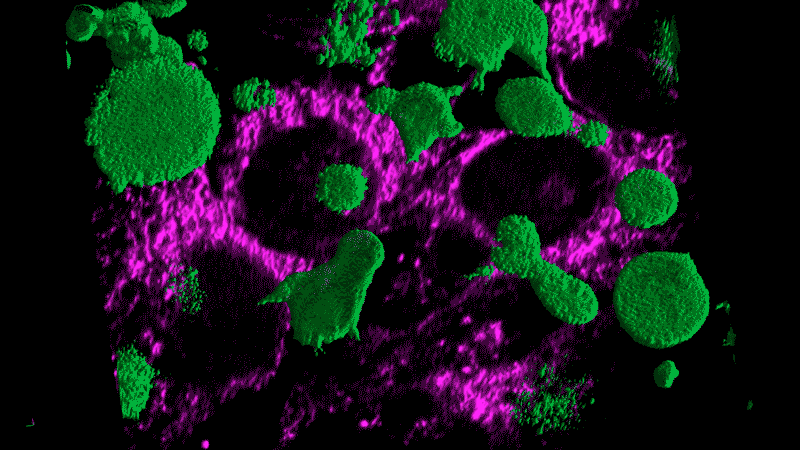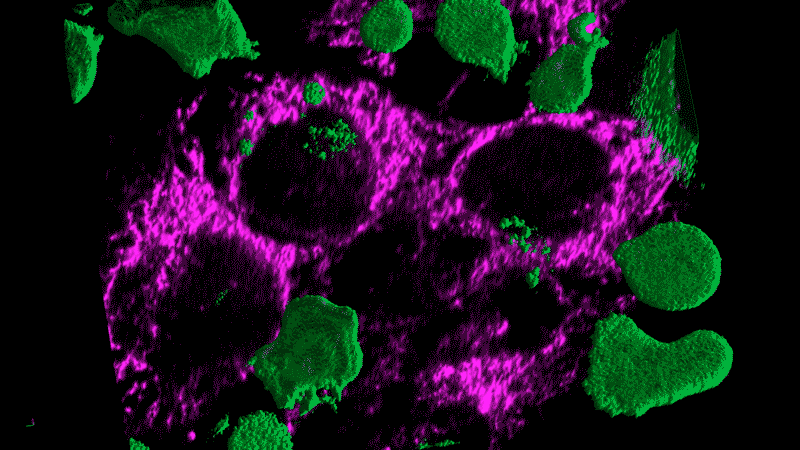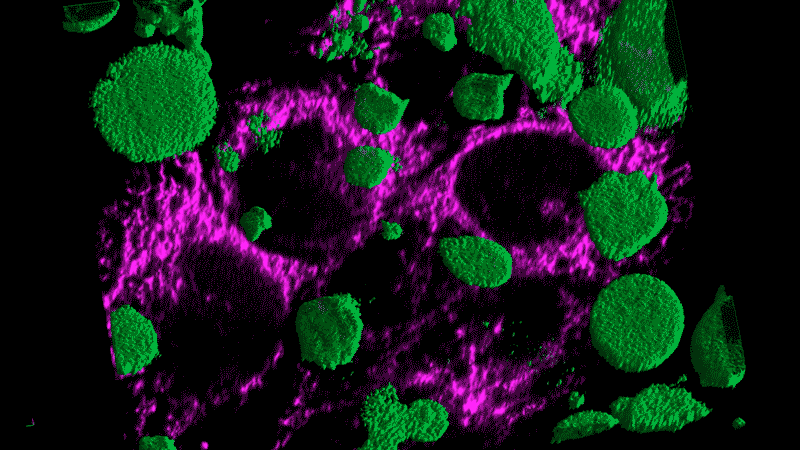
Researchers now think this kind of chronic inflammation could also fuel the flames of cancer, just as Virchow had suspected.
Tumor cells themselves appear as danger signals and can trigger inflammation. The resulting inflammation can end up – paradoxically – promoting tumor growth rather than inducing an assault on the tumor. The cancer cells persist and continue to appear as danger signals, initiating a vicious cycle.
Mounting evidence from a range of cancer studies suggests that this age-old hypothesis is worth attending to. What if chronic inflammation plays an important role in how to address tumor growth and progression? Novartis is committed to exploring the possibility that this approach could help patients with lung cancer and potentially other forms of cancer.
Learn why researchers are targeting #inflammation to fight #cancer.



